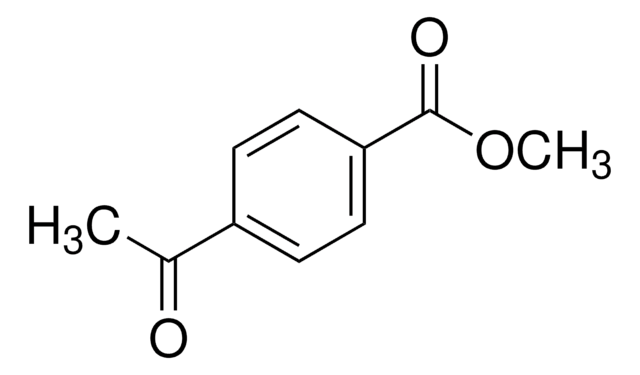
935395
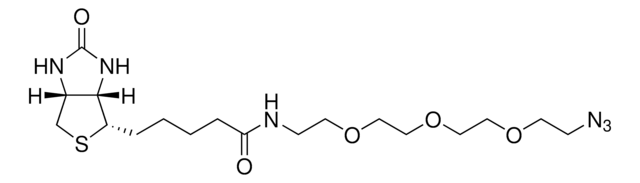
N-(3-Azidopropyl)biotinamide
≥95%
동의어(들):
(3aS,4S,6aR)-N-(3-Azidopropyl)hexahydro-2-oxo-1H-thieno[3,4-d]imidazole-4-pentanamide, 1H-Thieno[3,4-d]imidazole-4-pentanamide, N-(3-azidopropyl)hexahydro-2-oxo-, (3aS,4S,6aR)-, N-(3-Azidopropyl)biotinamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C13H22N6O2S
CAS Number:
Molecular Weight:
326.42
MDL number:
UNSPSC 코드:
12352125
NACRES:
NA.21
추천 제품
Quality Level
분석
≥95%
형태
solid
색상
white to beige
저장 온도
2-8°C
SMILES string
[N-]=[N+]=NCCCNC(=O)CCCCC1SCC2NC(=O)NC12
InChI
InChI=1S/C13H22N6O2S/c14-19-16-7-3-6-15-11(20)5-2-1-4-10-12-9(8-22-10)17-13(21)18-12/h9-10,12H,1-8H2,(H,15,20)(H2,17,18,21)/t9-,10-,12-/m0/s1
애플리케이션
This reagent enables the specific labeling of various alkynylated molecules, such as DNA, oligonucleotides, and proteins, with biotin. The binding of biotin to avidin or streptavidin can be employed in downstream affinity applications, such as the isolation of biotinylated molecules or their interaction with streptavidin conjugates. Biotin azide undergoes a copper-catalyzed click reaction with terminal alkynes, enabling the incorporation of biotin and biotin derivatives into biomolecules that contain alkyne groups through azide-alkyne cycloaddition.
특징 및 장점
Biotin-azide (N-(3-Azidopropyl)biotinamide) is an azido derived biotin probe. Biotin-azide can be used to prepare various biotinylated conjugates via Click Chemistry.The conjugation of biotin and its derivatives to various biomolecules can be achieved through the widely recognized click chemistry methodology, followed by their detection using streptavidin, avidin, or NeutrAvidin biotin-binding proteins. Biotin azide serves as a valuable reagent for the synthesis of diverse biotinylated conjugates via Click Chemistry
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Development of a Multifunctional Benzophenone Linker for Peptide Stapling and Photoaffinity Labelling
Wu Y, et al.
Chembiochem, 17, 689-692 (2016)
D C Montgomery et al.
Methods in enzymology, 574, 105-123 (2016-07-18)
Changes in reversible protein acetylation mediate many key aspects of genomic regulation and enzyme function. The catalysts for this posttranslational modification, lysine acetyltransferases (KATs), have been difficult targets for characterization due to their complex architecture and challenging reconstitution. To address
Christina M Woo et al.
Analytical and bioanalytical chemistry, 409(2), 579-588 (2016-10-04)
Protein glycosylation is a post-translational modification (PTM) responsible for many aspects of proteomic diversity and biological regulation. Assignment of intact glycan structures to specific protein attachment sites is a critical step towards elucidating the function encoded in the glycome. Previously
Kristina Hofmann et al.
Journal of lipid research, 55(3), 583-591 (2013-12-18)
Cholesterol is an important lipid of mammalian cells and plays a fundamental role in many biological processes. Its concentration in the various cellular membranes differs and is tightly regulated. Here, we present a novel alkyne cholesterol analog suitable for tracing
Qingfei Zheng et al.
The Journal of organic chemistry, 85(3), 1691-1697 (2019-12-26)
Methylglyoxal (MGO) is a reactive dicarbonyl metabolite that modifies histones in vivo and induces changes in chromatin structure and function. Here we report the synthesis and application of a chemical probe for investigating MGO-glycation. A two-step synthesis of a Cu-click
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.



![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)