추천 제품
Quality Level
분석
99%
형태
powder
mp
148-151 °C (lit.)
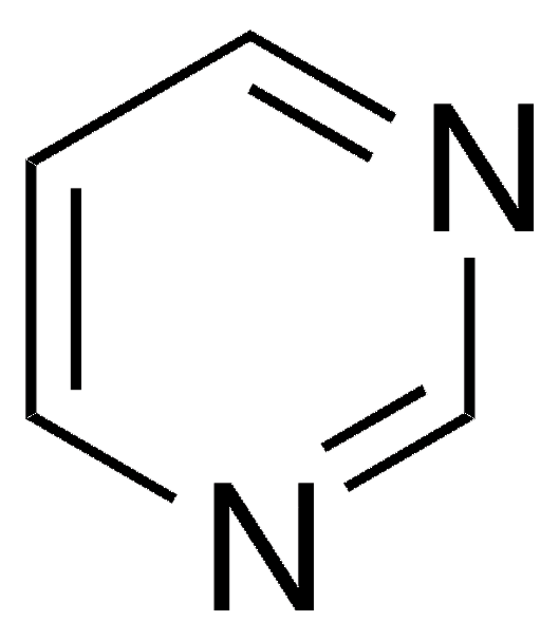
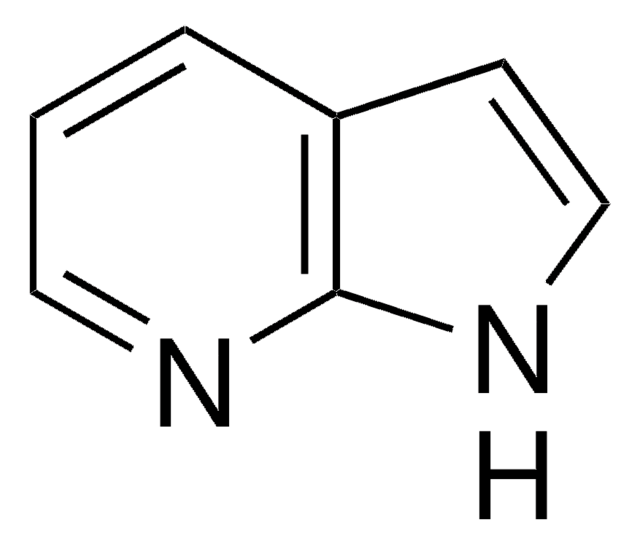
SMILES string
c1cnc2nc[nH]c2c1
InChI
1S/C6H5N3/c1-2-5-6(7-3-1)9-4-8-5/h1-4H,(H,7,8,9)
InChI key
GAMYYCRTACQSBR-UHFFFAOYSA-N
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Vassilios Bavetsias et al.
Bioorganic & medicinal chemistry letters, 17(23), 6567-6571 (2007-10-16)
A hit generation and exploration approach led to the discovery of 31 (2-(4-(6-chloro-2-(4-(dimethylamino)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)piperazin-1-yl)-N-(thiazol-2-yl)acetamide), a potent, novel inhibitor of Aurora-A, Aurora-B and Aurora-C kinases with IC(50) values of 0.042, 0.198 and 0.227microM, respectively. Compound 31 inhibits cell proliferation and has good
Dorte Renneberg et al.
Journal of the American Chemical Society, 125(19), 5707-5716 (2003-05-08)
The DNA binding properties of fused heterocycles imidazo[4,5-b]pyridine (Ip) and hydroxybenzimidazole (Hz) paired with pyrrole (Py) in eight-ring hairpin polyamides are reported. The recognition profile of Ip/Py and Hz/Py pairs were compared to the five-membered ring pairs Im/Py and Hp/Py
Andrea Cappelli et al.
Journal of medicinal chemistry, 49(22), 6451-6464 (2006-10-27)
The 4-phenylquinoline fragment of novel AT(1) receptor antagonists 4 based on imidazo[4,5-b]pyridine moiety was replaced by 4-phenylisoquinolinone (compounds 5) or 1-phenylindene (compounds 6) scaffolds to investigate the structure-activity relationships. Binding studies showed that most of the synthesized compounds display high
Ping Lan et al.
European journal of medicinal chemistry, 46(1), 77-94 (2010-11-26)
3D-QSAR and docking studies were performed on sixty imidazo[4,5-b]pyridine derivatives as Aurora A kinase inhibitors. The CoMFA and CoMSIA models using forthy-eight molecules in the training set, gave r(cv)(2) values of 0.774 and 0.800, r(2) values of 0.975 and 0.977
M J Wanner et al.
Nucleosides, nucleotides & nucleic acids, 23(8-9), 1313-1320 (2004-12-02)
Nitration of substituted (1-deaza)purines using a mixture of tetrabutylammonium nitrate (TBAN) and trifluoracetic acid anhydride (TFAA) was applied to prepare nitrosubstituted (1-deaza)purines at low temperature. The nitro group influences the system twofold: 1) it activates other substituents towards nucleophilic aromatic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![Imidazo[1,2-a]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/109/863/81ccb63f-07c6-4271-b317-1ba58979d455/640/81ccb63f-07c6-4271-b317-1ba58979d455.png)
![3-Methyl-3H-imidazo[4,5-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/309/037/0969ed2b-d1ce-49e9-87c8-ef436d41719a/640/0969ed2b-d1ce-49e9-87c8-ef436d41719a.png)

![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)