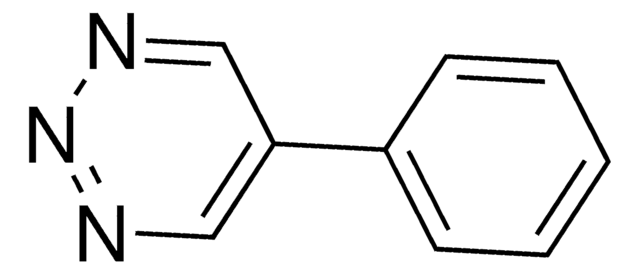
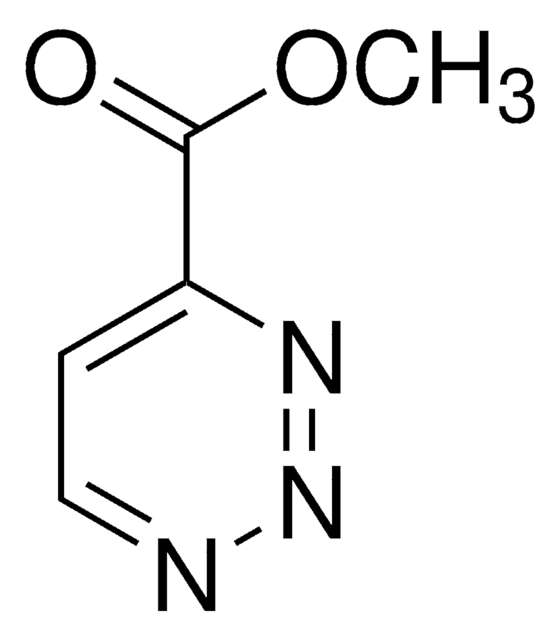
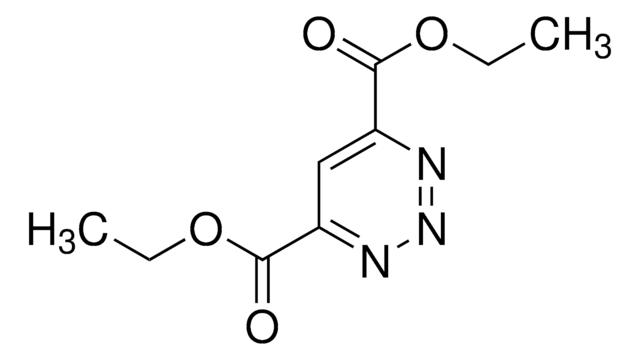
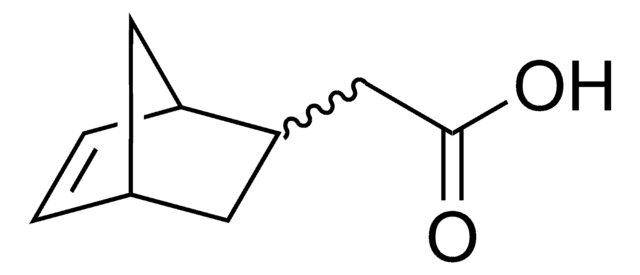
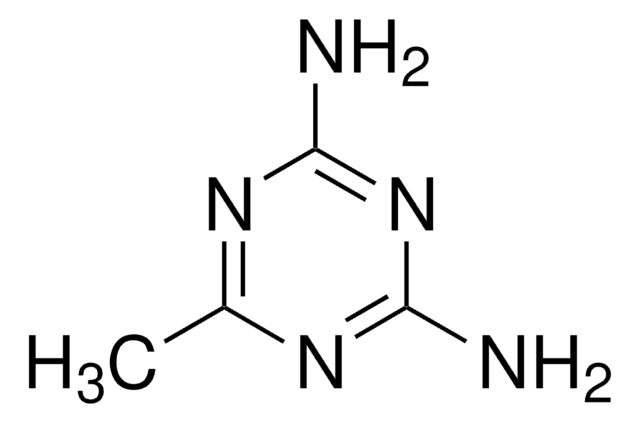
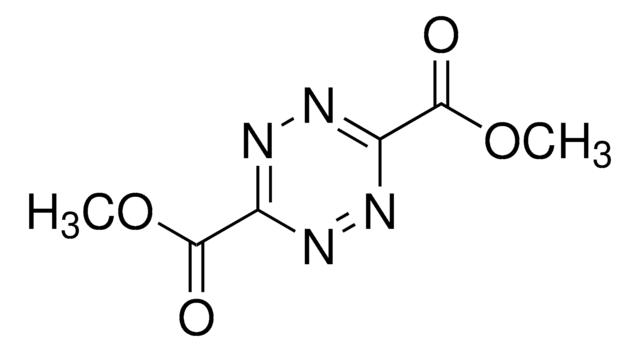
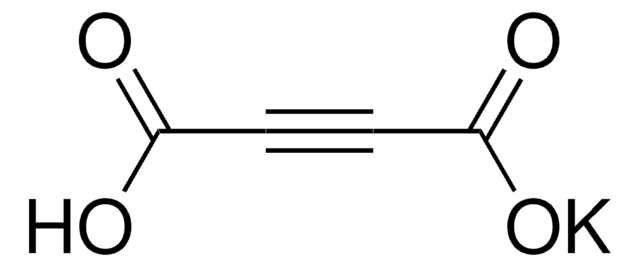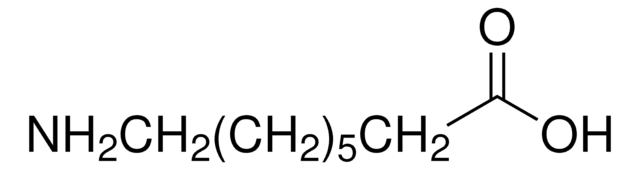추천 제품
형태
powder
Quality Level
저장 온도
2-8°C
SMILES string
COC1=CN=NN=C1
InChI
1S/C4H5N3O/c1-8-4-2-5-7-6-3-4/h2-3H,1H3
InChI key
HVBZCUMRMKODNE-UHFFFAOYSA-N
일반 설명
애플리케이션
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
문서
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
관련 콘텐츠
As the exploration of the properties of complex natural products becomes increasingly more sophisticated with the technological advances being made in their screening and evaluation and as structural details of their interaction with biological targets becomes more accessible, the importance and opportunities for providing unique solutions to complex biological problems has grown. The Boger Lab addresses these challenging problems by understanding the complex solutions and subtle design elements that nature has provided in the form of a natural product and work to extend the solution through rational design elements to provide more selective, more efficacious, or more potent agents designed specifically for the problem or target under investigation. The resulting efforts have reduced many difficult or intractable synthetic challenges to manageable problems providing an approach not only to the natural product but one capable of simple extrapolation to a series of structural analogs with improved selectivity and efficacy.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.