추천 제품
분석
98%
양식
powder
mp
112-114 °C (lit.)
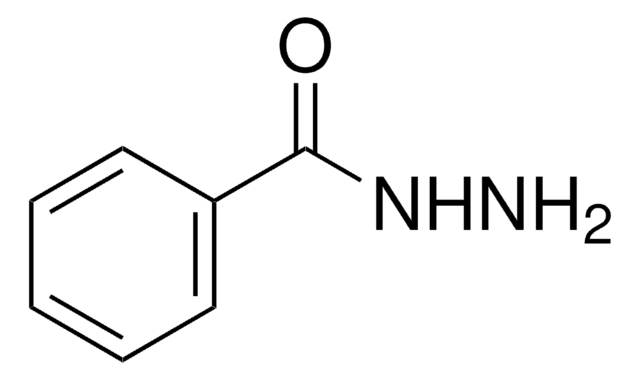
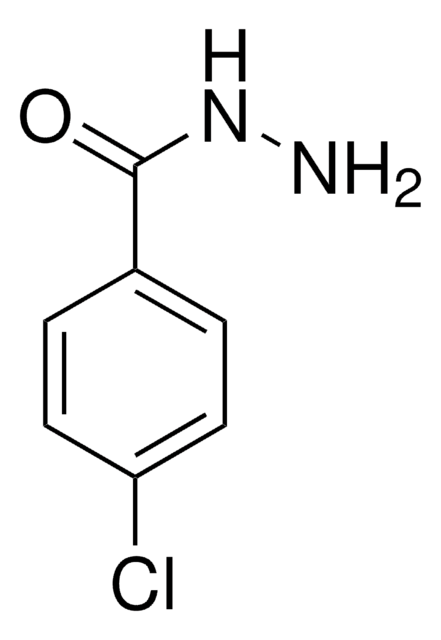
SMILES string
NNC(=O)c1ccccc1
InChI
1S/C7H8N2O/c8-9-7(10)6-4-2-1-3-5-6/h1-5H,8H2,(H,9,10)
InChI key
WARCRYXKINZHGQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
E Agostinelli et al.
The Biochemical journal, 324 ( Pt 2), 497-501 (1997-06-01)
A novel copper-depleted bovine serum amine oxidase (BSAO), in which about half the molecules contained the organic cofactor in the oxidized form, was prepared by adding a reductant in anaerobic conditions to the cyanide-reacted protein. The CuI-semiquinone formed in these
M Kobayashi et al.
Biochemical and biophysical research communications, 256(2), 415-418 (1999-03-18)
The amidase from Rhodococcus rhodochrous J1, which hydrolyses amide to acid and ammonia, was found to catalyze the synthesis of hydrazide using hydrazine as a substrate. This is the first report on the hydrazide synthesis through enzymatic reactions. The enzyme
Susan M Aitken et al.
The Biochemical journal, 375(Pt 3), 613-621 (2003-07-19)
Many compounds are oxidized by haem enzymes, such as peroxidases and cytochromes P450, to highly reactive intermediates that function as enzyme inactivators. To evaluate the potential of arylhydrazides as selective metabolically activated peroxidase inhibitors, the mechanism of HRPC (horseradish peroxidase
Venkataswamy Sorna et al.
Journal of medicinal chemistry, 56(23), 9496-9508 (2013-11-19)
Lysine specific demethylase 1 (LSD1) plays an important role in regulating histone lysine methylation at residues K4 and K9 on histone H3 and is an attractive therapeutic target in multiple malignancies. Here we report a structure-based virtual screen of a
Tetsuya Toya et al.
Bioorganic & medicinal chemistry, 10(4), 953-961 (2002-02-12)
We have investigated the biologically active conformation of the non-steroidal ecdysone agonist, 1-tert-butyl-1,2-dibenzoylhydrazine (RH-5849) by means of design, synthesis and conformational analysis of cyclic derivatives of RH-5849. Among the synthesized compounds, a 6-membered cyclic hydrazine bearing two benzoyl groups (5)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.