모든 사진(2)
About This Item
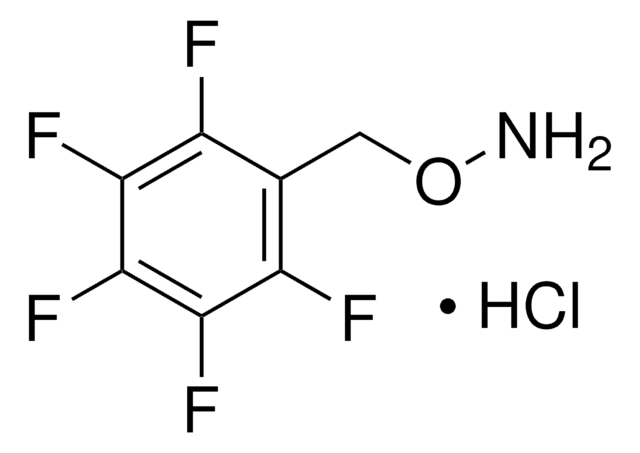
Linear Formula:
C6H5CH2ONH2 · HCl
CAS Number:
Molecular Weight:
159.61
Beilstein:
3687991
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Effective reagent used to prepare α-hydroxybenzylamines from α-hydroxyketones.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Tetrahedron Letters, 32, 711-711 (1991)
D F Magin
Journal of chromatography, 202(2), 255-261 (1980-12-19)
A qualitative and semi-quantitative method was established for the investigation of low-molecular-weight volatile carbonyl compounds in cigarette whole smoke. The carbonyls were trapped on a silica gel "column" and eluted with water. The aqueous solution was then treated with benzyloxyamine
Han Fu et al.
Journal of combinatorial chemistry, 9(5), 804-810 (2007-08-11)
A highly regioselective and traceless solid-phase route to 1,7,8-trisubstituted purines has been developed. This methodology could be extended to the preparation of 8-azapurines and [i]-condensed purines. A representative set of 17 purines, azapurines, and [i]-condensed purines was synthesized. This paper
G Cardillo et al.
Organic letters, 3(8), 1165-1167 (2001-05-12)
[reaction: see text]. The 1,4-addition of O-benzylhydroxylamine to alpha,beta-unsaturated imide 1 in the presence of BF3.Et2O proceeds with the preferential attack of the nucleophile on the Cbeta-re face. To explain this unexpected reactivity 1H, 13C, and 11B NMR investigations have
Yin Luo et al.
ChemMedChem, 7(9), 1587-1593 (2012-07-20)
Forty-three oxime derivatives were synthesized by allowing O-benzylhydroxylamines to react with primary benzaldehydes or salicylaldehydes; these products were gauged as potential inhibitors of β-ketoacyl-(acyl-carrier-protein) synthase III (FabH). Among the 43 compounds, 38 are reported herein for the first time. These
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.