추천 제품
형태
liquid
반응 적합성
reaction type: C-C Bond Formation
애플리케이션
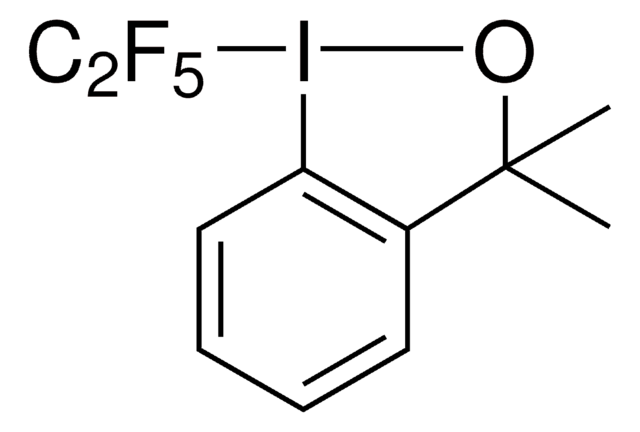
Phenylsulfanyltetrafluoroethyl bromide is a fluoroalkylbromide that is a radical source of the phenylsulfanyltetrafluoroethyl moiety. Alternatively, it can also be selectively metallated at the fluorinated carbon with Turbo Grignard reagent at low temperatures resulting in a thermally unstable anion that can act as a nucleophilic fluoroalkylation reagent towards a wide variety of electrophiles, such as aldehydes, ketones or sulfonylimines. A phenylsulfanyltetrafluoroethyl moiety incorporated in the substrate can be treated with tributyltin hydride and generate the corresponding fluoroalkyl radical. If the substrate lacks any olefins, it can be reduced to tetrafluoroethyl group whereas if the substrate contains an olefin in a correct spatial orientation, it can lead to an intramolecular cyclization affording tetrafluorinated cyclic structures.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
법적 정보
Product of CF Plus Chemicals.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 4 - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
문서
Fluoroalkylation toolbox expands with various reagents for late-stage fluoroalkylation in organic synthesis and medicinal chemistry.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.