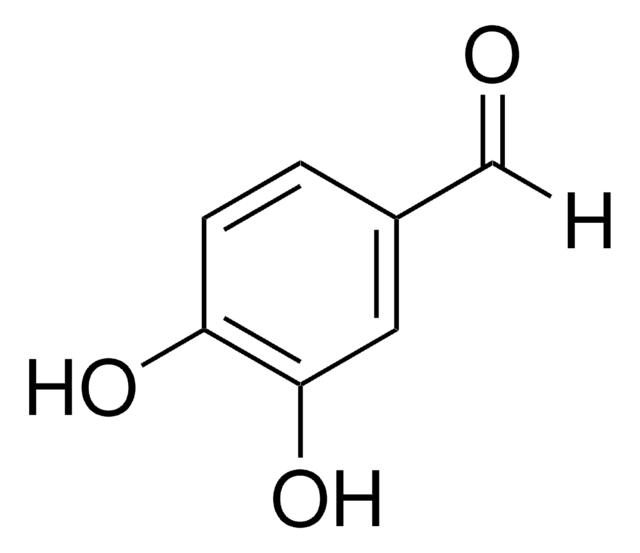
H19808
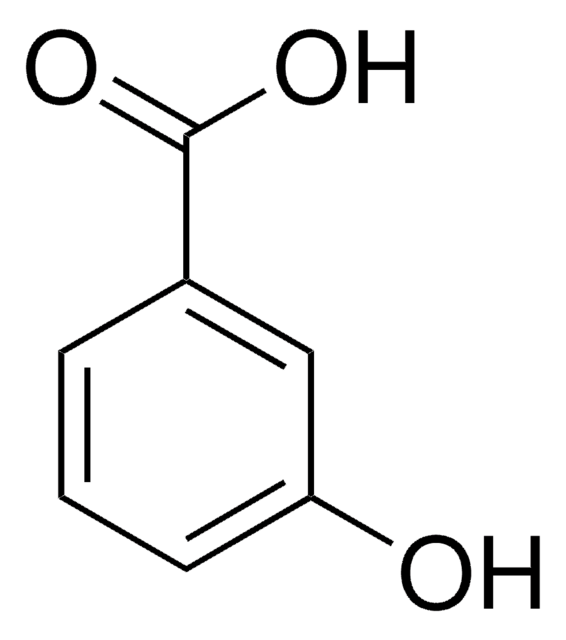
3-Hydroxybenzaldehyde
≥99%
동의어(들):
3-Formylphenol, 5-Hydroxybenzaldehyde, m-Formylphenol, m-Hydroxybenzaldehyde
로그인조직 및 계약 가격 보기
모든 사진(5)
About This Item
Linear Formula:
HOC6H4CHO
CAS Number:
Molecular Weight:
122.12
Beilstein:
507099
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥99%
bp
191 °C/50 mmHg (lit.)
mp
100-103 °C (lit.)
SMILES string
Oc1cccc(C=O)c1
InChI
1S/C7H6O2/c8-5-6-2-1-3-7(9)4-6/h1-5,9H
InChI key
IAVREABSGIHHMO-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
3-Hydroxybenzaldehyde can be used as a reactant along with ethyl acetoacetate and thiourea in the synthesis of corresponding dihydropyrimidine-2-thione (monastrol), using Yb(OTf)3 as a catalyst by Biginelli cyclocondensation reaction.
It can also be used as a starting material for the synthesis of:
It can also be used as a starting material for the synthesis of:
- (-)-Quinocarcin, isoxazolo[3,4-e][2,1]benzisoxazole and 3-n-propylphenol.
- Oligo-3-hydroxybenzaldehyde (O-3HBA) by oxidative polycondensation.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
The synthesis and thermal properties of oligo-3-hydroxybenzaldehyde synthesized by oxidative polycondensation
Mart H, et al.
Journal of Applied Polymer Science, 101(2), 892-897 (2006)
A novel cyclization to isoxazolo [3, 4-e][2, 1] benzisoxazole
Katritzky AR, et al.
Tetrahedron Letters, 43(18), 3449-3451 (2002)
Improved synthesis and preparative scale resolution of racemic monastrol
Dondoni A, et al.
Tetrahedron Letters, 43(34), 5913-5916 (2002)
A practical synthesis of 3-n-propylphenol, a component of tsetse fly attractant blends
Ujvary I and Mikite G
Organic Process Research & Development, 7(4), 585-587 (2003)
M Parekh et al.
Letters in applied microbiology, 22(2), 115-120 (1996-02-01)
Desulfovibrio desulfuricans ATCC 27774 was screened for reactivity against aromatic compounds during lactate-dependent, nitrate-dissimilating growth. Only aromatic aldehydes (benzaldehyde, 2-hydroxybenzaldehyde, 3-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, vanillin, iso-vanillin and o-vanillin) were reactive and, with the exception of 2-hydroxybenzaldehyde, were stimulatory to lactate-dependent growth. Aromatic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.