추천 제품
Quality Level
분석
≥99%
bp
220 °C (lit.)
mp
30-32 °C (lit.)
density
1.049 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI key
CUONGYYJJVDODC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- base-promoted on-water synthesis of [1,6]-naphthyridines.†
- synthesis of γ-ketoamides.
- preparation of heterocyclic privileged medicinal scaffolds involving pyridine, 1,4-dihydropyridine, chromeno[2,3-b]pyridine and dihydro-1,4-dithiepine frameworks.
포장
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
186.8 °F - closed cup
Flash Point (°C)
86 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
MOST offers controlled solar energy harvesting and storage, addressing global energy demands with improved storage techniques.
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
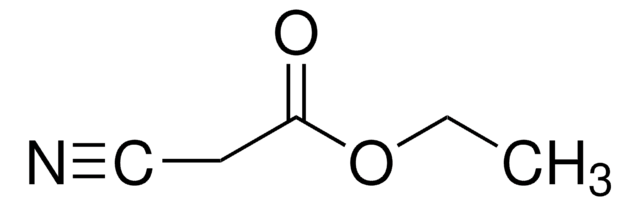
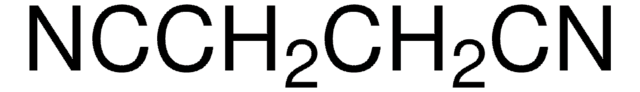



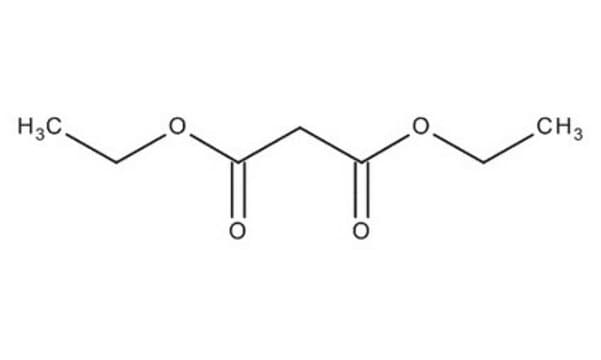

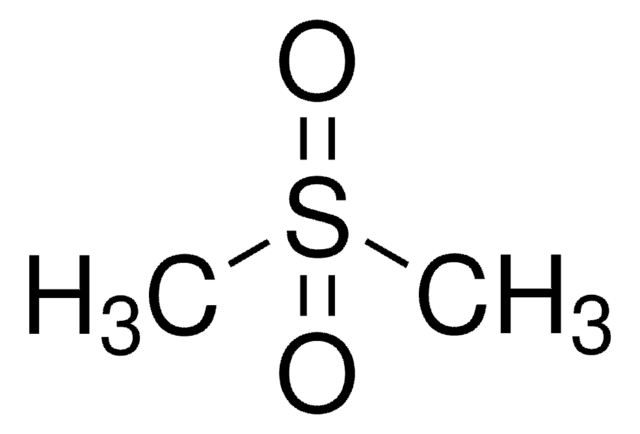



![2-[Bis(methylthio)methylene]malononitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)