추천 제품
분석
98%
형태
liquid
refractive index
n20/D 1.445 (lit.)
bp
118-119 °C/753 mmHg (lit.)
density
0.844 g/mL at 25 °C (lit.)
SMILES string
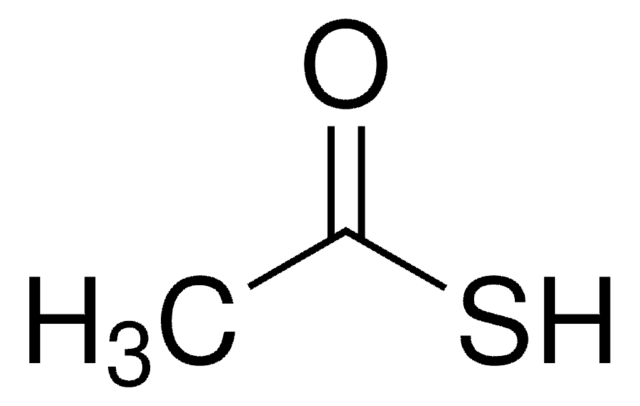
CC1CCCCN1
InChI
1S/C6H13N/c1-6-4-2-3-5-7-6/h6-7H,2-5H2,1H3
InChI key
NNWUEBIEOFQMSS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Reactant for C-2 arylation of piperidines through directed transition metal-catalyzed sp3 C-H activation
Reactant for synthesis of:
Reactant for synthesis of:
- Azepan-4-ones via two step [5+2] annulation
- 2-Aminobenzoxazoles
- Unsymmetrically substituted ureas
- Corticotropin-releasing factor receptor type 1 antagonists
- Gefitinib analogues with anti-tumor activity
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
50.0 °F - closed cup
Flash Point (°C)
10 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Dmitry Zuev et al.
Bioorganic & medicinal chemistry letters, 20(12), 3669-3674 (2010-05-18)
A novel series of [6-chloro-2-trifluoromethyl-7-aryl-7H-imidazo[1,2-a]imidazol-3-ylmethyl]-dialkylamines was discovered as potent CRF(1)R antagonists. The optimization of binding affinity in the series by the parallel reaction approach is discussed herein.
C-2 arylation of piperidines through directed transition-metal-catalyzed sp3 C-H activation.
Hana Prokopcová et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(44), 13063-13067 (2010-10-29)
Xiaoqing Wu et al.
Bioorganic & medicinal chemistry, 18(11), 3812-3822 (2010-05-15)
There is an urgent need to design and develop new and more potent EGFR inhibitors with improved anti-tumor activity. Here we describe the design and synthesis of two series of 4-benzothienyl amino quinazolines as new analogues of the EGFR inhibitor
Christopher L Cioffi et al.
The Journal of organic chemistry, 75(22), 7942-7945 (2010-10-27)
The synthesis of 2-aminobenzoxazoles can be readily achieved by two versatile, one-pot procedures utilizing commercially available tetramethyl orthocarbonate or 1,1-dichlorodiphenoxymethane, an amine, and an optionally substituted 2-aminophenol. The reactions were conducted under mild conditions and provided 2-aminobenzoxazoles in modest to
Li Cui et al.
Chemical communications (Cambridge, England), 46(19), 3351-3353 (2010-05-06)
A surprisingly efficient synthesis of azepan-4-ones via a two-step [5 + 2] annulation is developed. This reaction involves a key gold catalysis and shows generally high regioselectivities and good to excellent diastereoselectivities.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.