추천 제품
Quality Level
분석
96%
refractive index
n20/D 1.465 (lit.)
bp
88-91.5 °C (lit.)
density
1.065 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
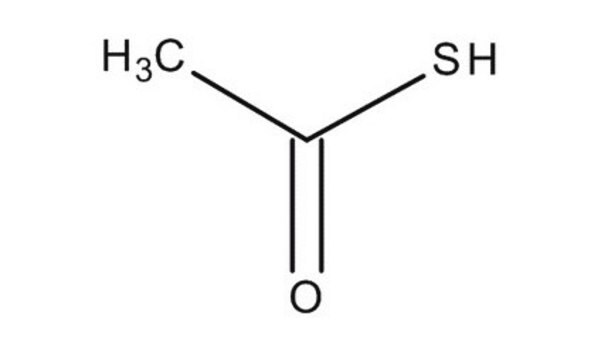
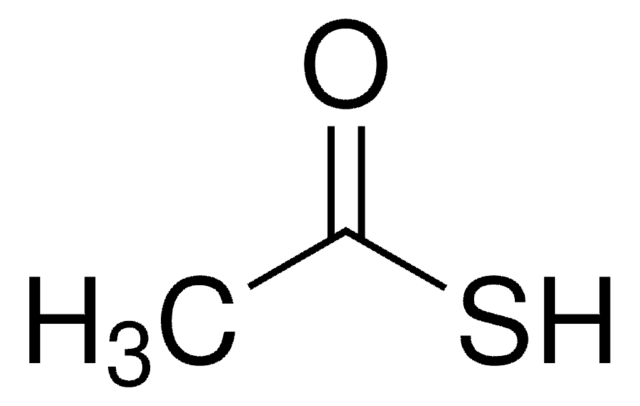
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
InChI key
DUYAAUVXQSMXQP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Thioacetic acid (TAA) can undergo:
- Enantioselective addition to nitroalkenes to form chiral 1,2-aminothiol derivatives in the presence of a novel sulfinyl urea organocatalyst. This method has been successfully employed in the synthesis of antifungal drug, sulconazole.
- Asymmetric Michael addition reaction with chalcones in the presence of a bifunctional amine thiourea catalyst to form synthetically useful thioesters.
- Asymmetric 1,6-conjugate addition with para-quinone methides in the presence of a chiral phosphoric acid catalyst to form chiral sulfur-containing diphenylmethane-type compounds.
- Conjugate addition to methacrylamides with chiral trans-2,5-disubstituted pyrrolidine auxiliaries to form chiral β-mercaptocarboxylic acid derivatives.
Thioacetic acid is a reagent for introduction of the thiol group into organic molecules.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
64.4 °F - closed cup
Flash Point (°C)
18 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Organocatalytic enantioselective Michael addition of thioacetic acid to enones.
Li Hao, et al.
Tetrahedron Letters, 47(18), 3145-3148 (2006)
Phosphoric Acid Catalyzed Asymmetric 1, 6?Conjugate Addition of Thioacetic Acid to para?Quinone Methides.
Dong N, et al.
Angewandte Chemie (International Edition in English), 55(4), 1460-1464 (2016)
Asymmetric induction in the conjugate addition of thioacetic acid to methacrylamides with chiral auxiliaries.
Kim B H, et al.
Tetrahedron Asymmetry, 16(6), 1215-1220 (2005)
I A Gol'dina et al.
Eksperimental'naia i klinicheskaia farmakologiia, 73(3), 25-27 (2010-04-23)
The influence of BM-7-02 compound on the production of cytokines in the culture of healthy volunteers' blood cells. This compound suppresses the production of main cytokine (gamma-IFN, IL-2 and IL-4) synthesized by CD4+ Th1 and Th2 cells, stimulates the production
Gita Sedghi et al.
Journal of the American Chemical Society, 130(27), 8582-8583 (2008-06-19)
A series of thioacetate-terminated butadiyne-linked porphyrin oligomers have been synthesized with one to three porphyrin repeat units. Single molecule electrical scanning tunneling microscopy measurements using the I(s) and I(t) methods were used to determine the molecule conductances for this series
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.