모든 사진(1)
About This Item
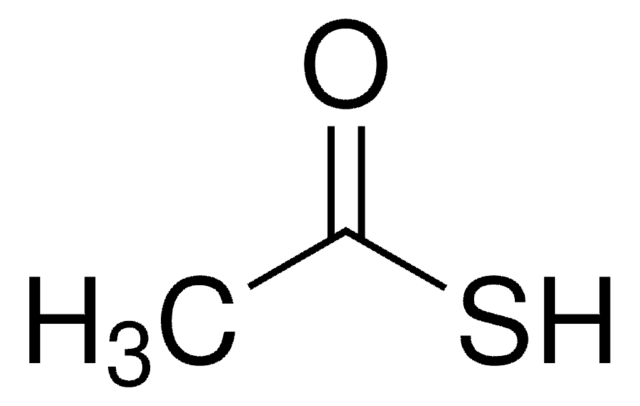
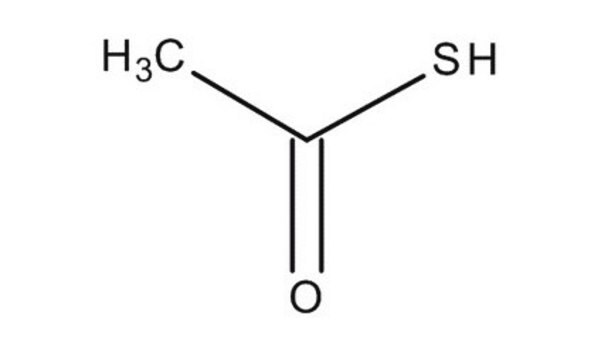
Linear Formula:
CH3COSH
CAS Number:
Molecular Weight:
76.12
FEMA Number:
4210
Beilstein:
1733298
EC Number:
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
12.199
NACRES:
NA.21
감각 수용성의:
meaty; roasted
생물학적 소스:
synthetic
식품 알레르기항원:
no known allergens
추천 제품
생물학적 소스
synthetic
Quality Level
분석
96%
refractive index
n20/D 1.465 (lit.)
bp
88-91.5 °C (lit.)
density
1.065 g/mL at 25 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
감각 수용성의
meaty; roasted
SMILES string
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
InChI key
DUYAAUVXQSMXQP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
면책조항
For R&D or non-EU Food use. Not for retail sale.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
64.4 °F - closed cup
Flash Point (°C)
18 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Krista M Wager et al.
Organic letters, 13(15), 4052-4055 (2011-07-07)
A method for preparing benzyl aryl thioethers utilizing an in situ deprotection of benzyl thioacetates as an alternative to free thiols as starting materials has been developed and optimized. Good to excellent yields of diverse benzyl aryl thioethers are obtained
Ning Shangguan et al.
Journal of the American Chemical Society, 125(26), 7754-7755 (2003-06-26)
A new amide synthesis strategy based on a fundamental mechanistic revision of the reaction of thio acids and organic azides is presented. The data demonstrate that amines are not formed as intermediates in this reaction. Alternative mechanisms proceeding through a
Sonia E Ulic et al.
The journal of physical chemistry. A, 112(27), 6211-6216 (2008-06-13)
Trifluorothioacetic acid-S-(trifluoromethyl)ester, CF3C(O)SCF3, was prepared by reacting CF3C(O)Cl and AgSCF3 at 50 degrees C. The compound was characterized by (13)C-, (19)F-NMR, UV, and vibrational spectroscopy as well as by gas electron diffraction (GED) and quantum chemical calculations (HF, MP2, and
Christina Wedemeyer-Exl et al.
Organic & biomolecular chemistry, 5(13), 2119-2128 (2007-06-22)
The thiol-dependent methylation of heptamethyl cob(II)yrinate 8r with methyl iodide and methyl tosylate was explored under a variety of conditions. The interaction of the heptamethyl cob(II)yrinate with a variety of thiols was monitored prior to the addition of the methylating
Md Ashraful Hoque et al.
Bioorganic & medicinal chemistry letters, 22(21), 6770-6772 (2012-10-02)
Two thioacetate tails were introduced to the chlamydocin- and CHAP31-related cyclic tetrapeptides. An intramolecular disulfide bridge could be formed in the CHAP31-related cyclic peptides. Both the thioacetate-tailed and disulfide-bridged peptides were potent histone deacetylase inhibitors in the presence of sulfhydryl
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.