241776
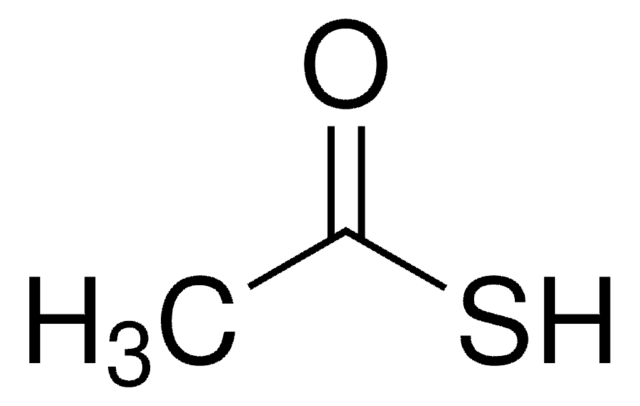
Potassium thioacetate
98%
동의어(들):
Ethanethioic acid potassium salt, Thioacetic acid potassium salt, Thiolacetic acid potassium salt
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
CH3COSK
CAS Number:
Molecular Weight:
114.21
Beilstein:
4428862
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
solid
mp
173-176 °C (lit.)
SMILES string
[K+].CC([S-])=O
InChI
1S/C2H4OS.K/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
InChI key
AFNBMGLGYSGFEZ-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Potassium thioacetate is a sulfur source in sulfuration reactions and is used as a reagent in nucleophilic substitution and vinylic substitution reactions.
애플리케이션
Palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives.
Reagent in the preparation of thiols from halides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Potassium thioacetate
Soria-Castro SM
Synlett (2012)
Potassium Thioacetate
Qiao Z and Jiang X
eEROS (Encyclopedia of Reagents for Organic Synthesis), 1-8 (2001)
Tetrahedron Letters, 48, 3033-3033 (2007)
Subal Dey et al.
Inorganic chemistry, 57(10), 5939-5947 (2018-05-02)
Reduction of CO2 holds the key to solving two major challenges taunting the society-clean energy and clean environment. There is an urgent need for the development of efficient non-noble metal-based catalysts that can reduce CO2 selectively and efficiently. Unfortunately, activation
Yang Wang et al.
Polymers, 11(6) (2019-06-13)
Photodynamic therapy (PDT) as a non-aggressive therapy with fewer side effects has unique advantages over traditional treatments. However, PDT still has certain limitations in clinical applications, mainly because most photosensitizers utilized in PDT are hydrophobic compounds, which will self-aggregate in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.