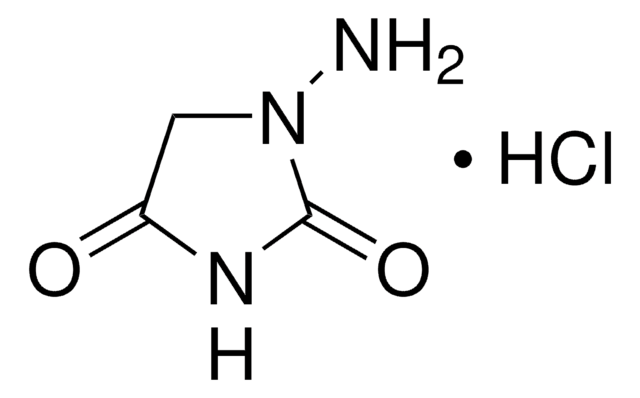
추천 제품
Quality Level
분석
≥99%
mp
175-177 °C (dec.) (lit.)
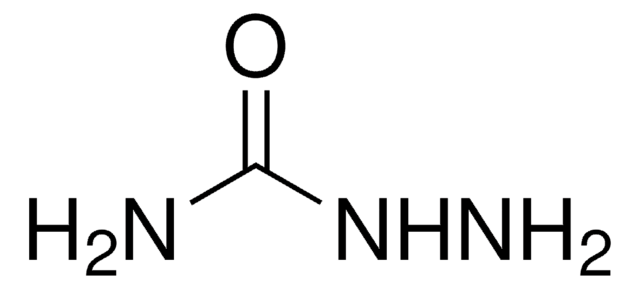
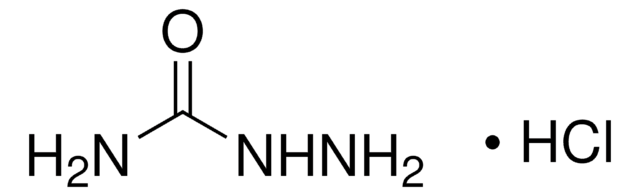
SMILES string
Cl.NNC(N)=O
InChI
1S/CH5N3O.ClH/c2-1(5)4-3;/h3H2,(H3,2,4,5);1H
InChI key
XHQYBDSXTDXSHY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Derivatization reagent for aldehydes and ketones which produces crystalline compounds with characteristic melting points.
Semicarbazide hydrochloride is a general reagent used to synthesize semicarbazones from aldehydes and ketones. It can be used to build a variety of heterocyclic compounds, some of which are potent antimicrobial and antiviral agents. It can also be used to prepare corrosion inhibitors.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Oral
표적 기관
Bone
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors.
Goulart C M, et al.
Corrosion Science, 67, 281-291 (2013)
Semicarbazide.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives.
Kirilmis C, et al.
European Journal of Medicinal Chemistry, 43(2), 300-308 (2008)
Design, synthesis, and biological evaluation of antiviral agents targeting flavivirus envelope proteins.
Li Z, et al.
Journal of Medicinal Chemistry, 51(15), 4660-4671 (2008)
Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives.
de Oliveira R B, et al.
European Journal of Medicinal Chemistry, 43(9), 1983-1988 (2008)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.