추천 제품
grade
puriss. p.a.
Quality Level
분석
98%
형태
crystals
무기 잔류물
≤0.08% (as SO4)
mp
180-183 °C (dec.) (lit.)
음이온 미량물
sulfate (SO42-): ≤100 mg/kg
양이온 미량물
Ca: ≤50 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
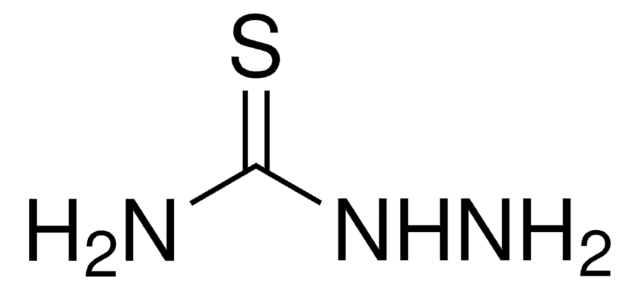
SMILES string
NNC(N)=S
InChI
1S/CH5N3S/c2-1(5)4-3/h3H2,(H3,2,4,5)
InChI key
BRWIZMBXBAOCCF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Thiosemicarbazide is an environmentally toxic compound, which is usually derived from thiourea. Its ability to chelate trace metals promotes its biological activity against certain tumors, protozoa, influenza, pesticides, and fungicides. It is widely used as a metal complexing agent in various fields involving the characterization of aliphatic or aromatic aldehydes, ketones, and polysaccharides.
애플리케이션
Thiosemicarbazide has been used as an analytical reagent for the measurement of urea net high-affinity uptake in roots of intact maize plants using colorimetric assay.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Aquatic Chronic 3
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Electrochemical determination of thiosemicarbazide using the glassy carbon electrode modified with multi-walled carbon nanotubes
Faizbakhsh N and Safari Z
Advances in Nanochemistry, 1(2), 52-55 (2019)
Measurement of net high-affinity urea uptake in maize plants
Zanin L, et al.
Bio-protocol, 5 (2015)
Juan Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 93, 245-249 (2012-04-10)
A simple and highly selective colorimetric sensor (L1) bearing thiosemicarbazide moiety as binding site and nitrophenyl moiety as signal group were synthesized. Sensor L1 showed great colorimetric single selectivity and high sensitivity for mercury cation in DMSO and DMSO/H(2)O binary
Agata Siwek et al.
Journal of molecular modeling, 19(3), 1319-1324 (2012-11-29)
The preliminary cytotoxic effect of 4-ethoxycarbonylmethyl-1-(piperidin-4-ylcarbonyl)-thiosemicarbazide hydrochloride (1)-a potent topoisomerase II inhibitor-was measured using a MTT assay. It was found that the compound decreased the number of viable cells in both estrogen receptor-positive MCF-7 and estrogen receptor-negative MDA-MB-231breast cancer cells
Pedro I da S Maia et al.
Inorganic chemistry, 51(3), 1604-1613 (2012-01-12)
Na[AuCl(4)]·2H(2)O reacts with tridentate thiosemicarbazide ligands, H(2)L1, derived from N-[N',N'-dialkylamino(thiocarbonyl)]benzimidoyl chloride and thiosemicarbazides under formation of air-stable, green [AuCl(L1)] complexes. The organic ligands coordinate in a planar SNS coordination mode. Small amounts of gold(I) complexes of the composition [AuCl(L3)] are
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.