추천 제품
형태
powder
포장
pkg of 1 × 10 mg (890701P-10mg)
pkg of 1 × 25 mg (890701P-25mg)
제조업체/상표
Avanti Research™ - A Croda Brand
지질 유형
transfection
cationic lipids
배송 상태
dry ice
저장 온도
−20°C
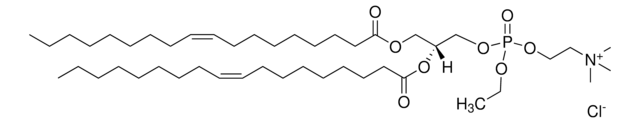
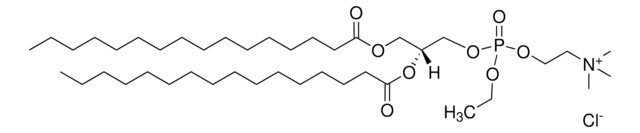
SMILES string
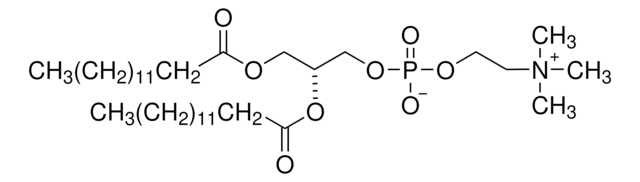
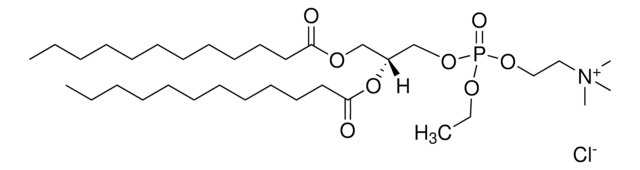
O=P(OCC[N+](C)(C)C)(OC[C@]([H])(OC(CCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCC)=O)OCC.[Cl-]
일반 설명
1,2-dimyristoyl-sn-glycero-3-ethylphosphocholine (14:0 EPC) is an acyl cationic lipid. O-alkyl phosphatidylcholines constitute the first chemically stable tri-esters of biological lipid structures and the first cationic derivatives of phospholipids consisting entirely of biological metabolites linked with ester bonds. The lipid has low toxicity and is biodegradable.
애플리케이션
1,2-dimyristoyl-sn-glycero-3-ethylphosphocholine (14:0 EPC (Cl Salt)) is suitable for use in liposome formulations.
생화학적/생리학적 작용
Synthetic cationic lipids serve as non-viral gene delivery agents. Change in the hydrocarbon chain of phosphatidylcholine (PC) derivatives affects transfection efficiency. 1,2-dimyristoyl-sn-glycero-3-ethylphosphocholine (14:0 EPC) aids in hydration during the formation of liposomes.
포장
5 mL Clear Glass Sealed Ampule (890701P-10mg)
5 mL Clear Glass Sealed Ampule (890701P-25mg)
법적 정보
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
William P D Goldring et al.
Bioorganic & medicinal chemistry letters, 22(14), 4686-4692 (2012-06-19)
The synthesis and in vitro evaluation of four cationic lipid gene delivery vectors, characterized by acyclic or macrocyclic, and saturated or unsaturated hydrophobic regions, is described. The synthesis employed standard protocols, including ring-closing metathesis for macrocyclic lipid construction. All lipoplexes
Paria Parvizi et al.
International journal of pharmaceutics, 461(1-2), 145-156 (2013-12-04)
This study seeks correlations between the molecular structures of cationic and neutral lipids, the lipid phase behavior of the mixed-lipid lipoplexes they form with plasmid DNA, and the transfection efficacy of the lipoplexes. Synthetic cationic pyridinium lipids were co-formulated (1:1)
Emile Jubeli et al.
European journal of medicinal chemistry, 125, 225-232 (2016-09-24)
In this communication we describe the construction of four succinic-based cationic lipids, their formulation with plasmid DNA (pDNA), and an evaluation of their in vitro gene delivery into Chinese hamster ovarian (CHO-K1) cells. The cationic lipids employed in this work possess
Kervin O Evans et al.
Chemistry and physics of lipids, 220, 49-56 (2019-02-24)
The capacity of molecules to inhibit oxidation is widely tested using liposomes as host matrices of the antioxidant molecule of interest. Spectroscopic assays are readily used for this purpose, specifically assays using 2,2'-azobis(2-methylpropionamidine) dihydrochloride (AAPH). In this work the effect
Rumiana Koynova et al.
The journal of physical chemistry. B, 111(27), 7786-7795 (2007-06-19)
Some mixtures of two cationic lipids including phospholipid compounds (O-ethylphosphatidylcholines) as well as common, commercially available cationic lipids, such as dimethylammonium bromides and trimethylammonium propanes, deliver therapeutic DNA considerably more efficiently than do the separate molecules. In an effort to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.