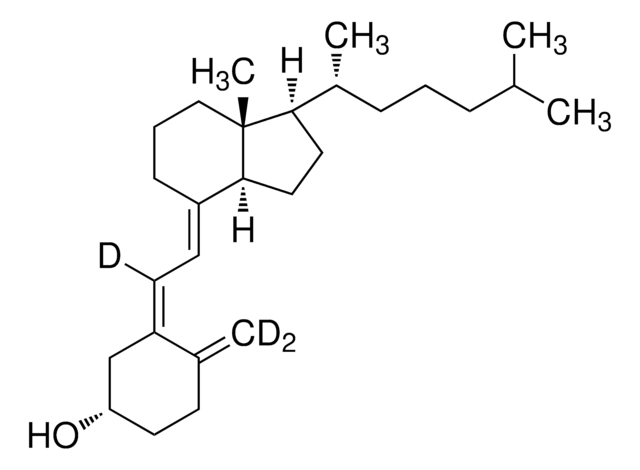
5.00936
Vitamin D₃ (cholecalciferol) cryst. (1 g = 40 Mio.I.U.)
EMPROVE® ESSENTIAL, Ph. Eur., BP, USP
의약품 제조
동의어(들):
Vitamin D₃ (cholecalciferol) cryst. (1 g = 40 Mio.I.U.), Colecalciferol, Cholecalciferol
About This Item
추천 제품
Agency
BP
Ph. Eur.
USP
Quality Level
제품 라인
EMPROVE® ESSENTIAL
양식
solid
mp
82-87 °C
solubility
<0.1 g/L
응용 분야
liquid formulation
pharmaceutical
저장 온도
2-8°C
SMILES string
O[C@H]1CCC(=C)\C(=C\C=C2\[C@H]3[C@@]([C@H](CC3)[C@@H](CCCC(C)C)C)(CCC\2)C)\C1
InChI
1S/C27H44O/c1-19(2)8-6-9-21(4)25-15-16-26-22(10-7-17-27(25,26)5)12-13-23-18-24(28)14-11-20(23)3/h12-13,19,21,24-26,28H,3,6-11,14-18H2,1-2,4-5H3/b22-12+,23-13+/t21-,24+,25-,26+,27-/m1/s1
InChI key
QYSXJUFSXHHAJI-FVUVGDFOSA-N
일반 설명
애플리케이션
법적 정보
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - STOT RE 1 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Medicine for children poses unique formulation challenges compared to adults. Consider developmental physiology and age specifics when designing pharmaceuticals. Quality issues can severely impact patient safety. Therefore, excipient quality, supplier selection, and supply chain security are crucial, particularly for pediatric formulations.
관련 콘텐츠
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.