5.05602
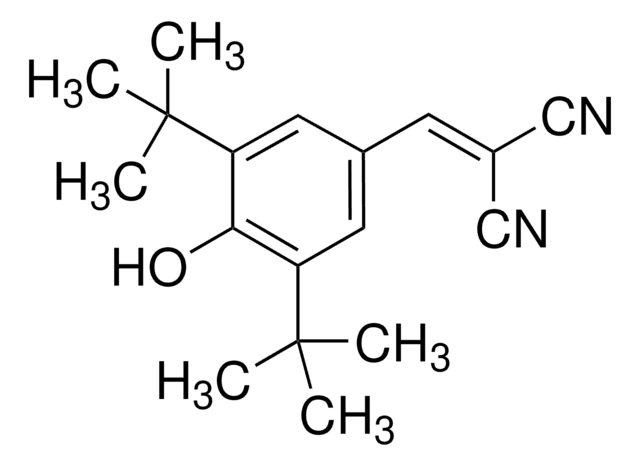
BDK Inhibitor II, (S)-CPP
동의어(들):
BDK Inhibitor II, (S)-CPP, (S)-α-Chloro-phenylpropionic acid, BCKD Kinase Inhibitor II, Branched-Chain α-Ketoacid Dehydrogenase Kinase Inhibitor II, BCKD Kinase Inhibitor II, Branched-Chain α-Ketoacid Dehydrogenase Kinase Inhibitor II, (S)-α-Chloro-phenylpropionic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
추천 제품
분석
≥98% (HPLC)
Quality Level
형태
oil
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
desiccated
protect from light
색상
clear colorless
solubility
DMSO: 100 mg/mL
저장 온도
−20°C
일반 설명
A cell-permeable chlorophenylpropionate compound that is superior to its structural analog 4PB (Cat. No. 567616) as an inhibitor against mitochondrial branched-chain α-ketoacid dehydrogenase complex (BCKDC) regulatory kinase BDK (BCKD kinase; IC50 = 6.3 vs. 53.1 M) due to much optimized affinity toward the BDK N-terminal allosteric site (Kd = 2.4 vs. 5.7 M), effectively blocking BDK substrate access by preventing BDK interaction with the homo-24-meric dihydrolipoyltransferase BCKDC E2 component, while exhibiting little potency against PDK2 or BCKD phosphatase BDP even at concentrations as high as 1 mM (<10% inhibition). Shown to effectively reduce E1-α Ser293 phosphorylation with concomitant BCKDC activity upregulation in multiple tissues in mice (EDmax =160 mg/kg via i.p.) in vivo. Despite good stability and low in vivo clearance , high doses are recommended for mice treatment due to low tissue penetration efficiency.
A cell-permeable chlorophenylpropionate compound that is superior to its structural analog 4PB (Cat. No. 567616) as an inhibitor against mitochondrial BCKDC (branched-chain α-ketoacid dehydrogenase complex) regulatory kinase BDK (BCKD kinase; IC50 = 6.3 vs. 53.1 µM) due to much optimized affinity toward the BDK N-terminal allosteric site (Kd = 2.4 vs. 5.7 µM) via carboxylate oxygens-mediated hydrogen bonds, as well as hydrophobic interactions mediated by the α-chlorine and the phenyl ring, effectively blocking BDK substrate access by preventing BDK interaction with the homo-24-meric dihydrolipoyltransferase BCKDC E2 component, while exhibiting little potency against PDK2 (pyruvate dehydrogenase kinase isoform 2) or BDP (BCKD phosphatase) even at concentrations as high as 1 mM (<10% inhibition). Shown to effectively reduce E1-α Ser293 phosphorylation with concomitant BCKDC activity upregulation in multiple tissues in mice (EDmax =160 mg/kg via i.p.; 60 min) in vivo, resulting in significant downregulation of serum BCAA (branched-chain amino acid) Leu/Ile and Val levels. Pharmacokinetic studies reveal good stability in vitro (half-life = 3.1 h and 6.8 h using murine liver S9 preparation or murine hepatocytes, respectively) and low clearance in vivo (CL/F = 0.113 mL/min; 40 mg/kg i.p.), however the compound does not penetrate tissues efficiently (VZ/F = 20.8 mL; 40 mg/kg i.p.) and high dosages are recommended for mice treatment.
생화학적/생리학적 작용
Cell permeable: yes
Primary Target
BDK
BDK
Reversible: yes
포장
Packaged under inert gas
경고
Toxicity: Standard Handling (A)
재구성
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 1 month at -20°C.
Use only fresh DMSO for reconstitution.
기타 정보
Tso, S.C., et al. 2013. Proc. Natl. Acad. Sci. USA110, 9728.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.