추천 제품
제품명
2-Chlorotrityl chloride resin (100-200 mesh), 1% DVB, Novabiochem®
Quality Level
제품 라인
Novabiochem®
양식
beads
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
응용 분야
peptide synthesis
저장 온도
2-30°C
일반 설명
2-Chlorotrityl chloride resin is an extremely versatile, acid-labile resin for the solid phase immobilization of carboxylic acids [1,2,3,4,5,6, 20], alcohols [7,8,9, 20], phenols [10,11,12,13, 20] and amines [14,15,16,17,18,19,20], imidazoles [20] and hydroxylamines [21,22]. Release of these functionalities is generally achieved using 1-50% TFA in DCM containing 5% TIS, although carboxylic acids can also be cleaved from this support with AcOH/TFE/DCM [1,2,3,4,5,6],0.5% TFA in DCM, or HFIP in DCM [23].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis ResinsLiterature references
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3943.
[3] K. Barlos, et al. (1991) Angew. Chem. Int. Ed. Engl., 30, 590.
[4] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 37, 513.
[5] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 562.
[6] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 555.
[7] H. Wenschuh, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1995, pp. 287.
[8] H. Wenschuh, et al. (1995) J. Org. Chem., 60, 405.
[9] B. B. Shankar, et al. (1998) Tetrahedron Lett., 39, 2447.
[10] Z. Zhu & B. Mckittrick (1998) Tetrahedron Lett., 39, 7479.
[11] U. Heinelt, et al. (2001) Bioorg. Med. Chem. Lett., 11, 227.
[12] K. J. Elgie, et al. (2000) Tetrahedron Lett., 41, 2753.
[13] S. Batra, et al. (2000) Tetrahedron Lett., 41, 5971.
[14] M. Cardno, et al. (1995) J. Chem. Soc., Chem. Commun., 2163.
[15] I. A. Nash, et al. (1996) Tetrahedron Lett., 37, 2625.
[16] W. J. Hoekstra, et al. (1997) Tetrahedron Lett., 38, 2629.
[17] J. Perumattan, et al. (1998) Mol. Div., 3, 121.
[18] J. J. McNally, et al. (1998) Tetrahedron Lett., 39,967.
[19] M. A. Youngman & S. L. Dax (1997) Tetrahedron Lett., 38, 6347.
[20] A. Bernhardt, et al. (1997) J. Peptide Res., 50, 143.
[21] S. L. Mellor, et al. (1997) Tetrahedron Lett., 38, 3311.
[22] M. M. Meloni & M. Taddei (2001) Org. Lett., 3, 337.
[23] R. Bollhagen, et al. (1994) J. Chem. Soc ., Chem. Commun., 2559.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis ResinsLiterature references
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3943.
[3] K. Barlos, et al. (1991) Angew. Chem. Int. Ed. Engl., 30, 590.
[4] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 37, 513.
[5] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 562.
[6] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 555.
[7] H. Wenschuh, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1995, pp. 287.
[8] H. Wenschuh, et al. (1995) J. Org. Chem., 60, 405.
[9] B. B. Shankar, et al. (1998) Tetrahedron Lett., 39, 2447.
[10] Z. Zhu & B. Mckittrick (1998) Tetrahedron Lett., 39, 7479.
[11] U. Heinelt, et al. (2001) Bioorg. Med. Chem. Lett., 11, 227.
[12] K. J. Elgie, et al. (2000) Tetrahedron Lett., 41, 2753.
[13] S. Batra, et al. (2000) Tetrahedron Lett., 41, 5971.
[14] M. Cardno, et al. (1995) J. Chem. Soc., Chem. Commun., 2163.
[15] I. A. Nash, et al. (1996) Tetrahedron Lett., 37, 2625.
[16] W. J. Hoekstra, et al. (1997) Tetrahedron Lett., 38, 2629.
[17] J. Perumattan, et al. (1998) Mol. Div., 3, 121.
[18] J. J. McNally, et al. (1998) Tetrahedron Lett., 39,967.
[19] M. A. Youngman & S. L. Dax (1997) Tetrahedron Lett., 38, 6347.
[20] A. Bernhardt, et al. (1997) J. Peptide Res., 50, 143.
[21] S. L. Mellor, et al. (1997) Tetrahedron Lett., 38, 3311.
[22] M. M. Meloni & M. Taddei (2001) Org. Lett., 3, 337.
[23] R. Bollhagen, et al. (1994) J. Chem. Soc ., Chem. Commun., 2559.
애플리케이션
- Efficient Synthesis of Protein Mimics by Sequential Native Chemical Ligation: This study utilized 2-Chlorotrityl chloride resin (100-200 mesh, 1% DVB) for solid-phase peptide synthesis, highlighting its effective use in complex peptide assembly processes. (Kruijtzer & Liskamp).
결합
Replaces: 01-64-0114
분석 메모
Colour (visual): white to yellow to beige
Appearance of substance (visual): beads
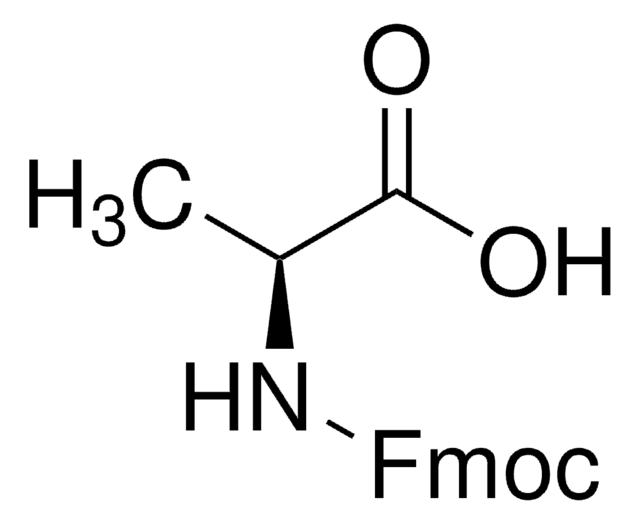
Loading (determined from the substitution of the Fmoc-Ala-Leu loaded resin): 1.00 - 1.80 mmol/g
Swelling Volume (in CH₂Cl₂): 2.0 - 5.0 ml/g
Total swelling volume acc. Houben Weyl (in CH2Cl2): ≥ 4.2 ml/g
The polymer matrix is copoly (styrene-1 % DVB) 100 - 200 mesh.
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Ala-Leu loaded resin): 1.00 - 1.80 mmol/g
Swelling Volume (in CH₂Cl₂): 2.0 - 5.0 ml/g
Total swelling volume acc. Houben Weyl (in CH2Cl2): ≥ 4.2 ml/g
The polymer matrix is copoly (styrene-1 % DVB) 100 - 200 mesh.
법적 정보
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Novabiochem® offers a wide range of linkers and derivatized resins for Fmoc solid-phase peptide synthesis with specialized protocols.
프로토콜
Review various resins like Merrifield, trityl-based, and hydroxymethyl-functionalized for peptide immobilization for diverse applications.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.