추천 제품
product name
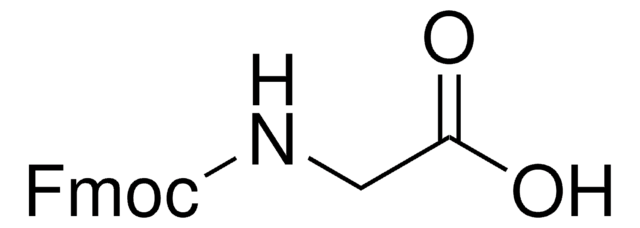
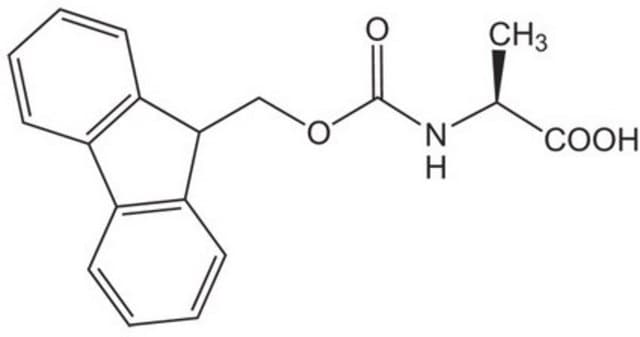
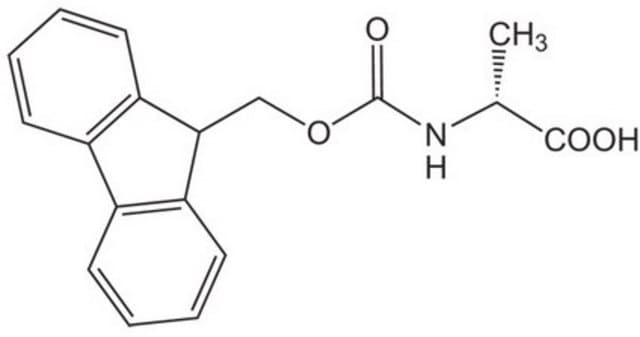
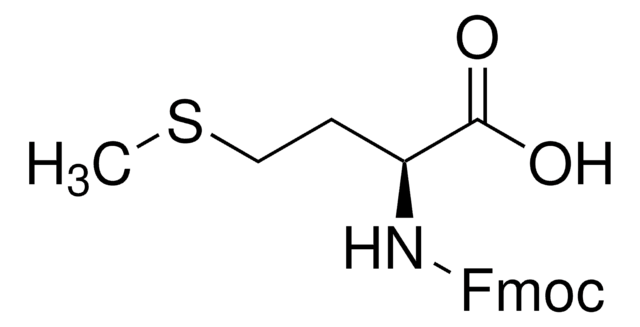
Fmoc-Ala-OH, 95%
Quality Level
분석
95%
광학 활성
[α]20/D −18°, c = 1 in DMF
반응 적합성
reaction type: C-H Activation
reaction type: Fmoc solid-phase peptide synthesis
reagent type: ligand
reaction type: Peptide Synthesis
mp
147-153 °C (lit.)
응용 분야
peptide synthesis
작용기
Fmoc
amine
carboxylic acid
저장 온도
2-8°C
SMILES string
C[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C18H17NO4/c1-11(17(20)21)19-18(22)23-10-16-14-8-4-2-6-12(14)13-7-3-5-9-15(13)16/h2-9,11,16H,10H2,1H3,(H,19,22)(H,20,21)/t11-/m0/s1
InChI key
QWXZOFZKSQXPDC-NSHDSACASA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Fmoc-Ala-OH also known as Fmoc-L-alanine, is a versatile reagent used in Fmoc solid-phase peptide synthesis.
애플리케이션
Fmoc-Ala-OH is commonly used :
- as a building block in the preparation of triazolopeptides , and azapeptides
- in the synthesis of bis-cationic porphyrin peptides using the standard Fmoc solid-phase synthesis
- to transform Mannich-adducts into α-halogenated amides without undergoing aziridination
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
? -Halo amides as competent latent enolates: direct catalytic asymmetric Mannich-type reaction
B Sun
Journal of the American Chemical Society, 139, 8295-8301 (2017)
Substrate-derived triazolo-and azapeptides as inhibitors of cathepsins K and S
M Galibert
European Journal of Medicinal Chemistry, 144, 201-210 (2018)
Towards sequence selective DNA binding: design, synthesis and DNA binding studies of novel bis-porphyrin peptidic nanostructures
E Biron
Organic & Biomolecular Chemistry, 6, 2507-2515 (2008)
Fabian Schnitter et al.
Nature protocols, 16(8), 3901-3932 (2021-07-02)
Many supramolecular materials in biological systems are driven to a nonequilibrium state by the irreversible consumption of high-energy molecules such as ATP or GTP. As a result, they exhibit unique dynamic properties such as a tunable lifetime, adaptivity or the
Xue Zhi Zhao et al.
Molecules (Basel, Switzerland), 23(8) (2018-07-28)
HIV-1 integrase (IN) inhibitors represent a new class of highly effective anti-AIDS therapeutics. Current FDA-approved IN strand transfer inhibitors (INSTIs) share a common mechanism of action that involves chelation of catalytic divalent metal ions. However, the emergence of IN mutants
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.