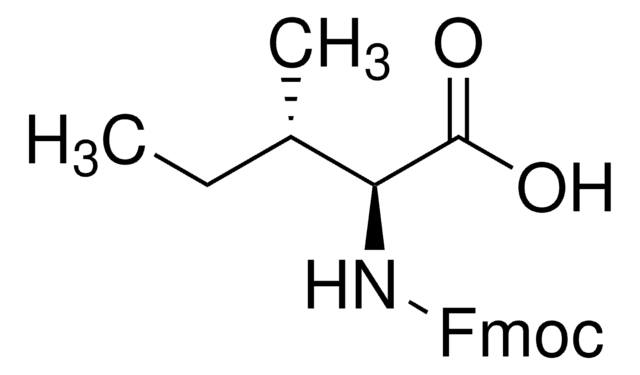
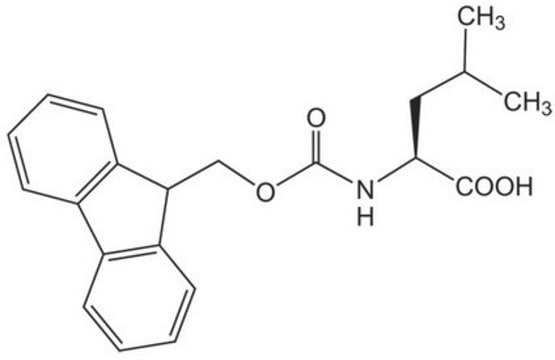
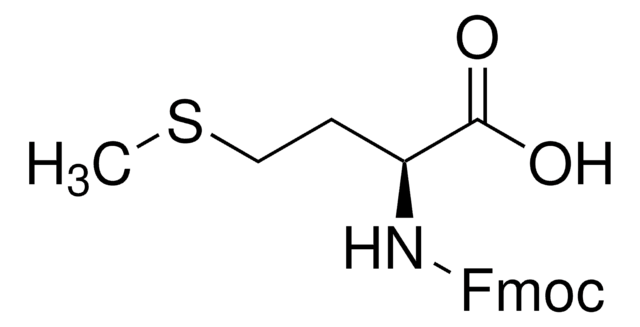
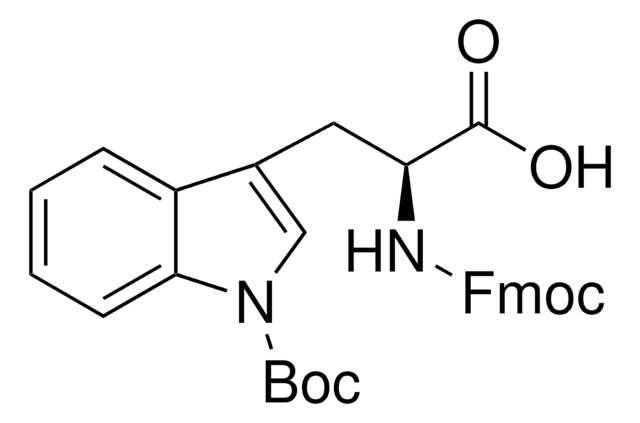
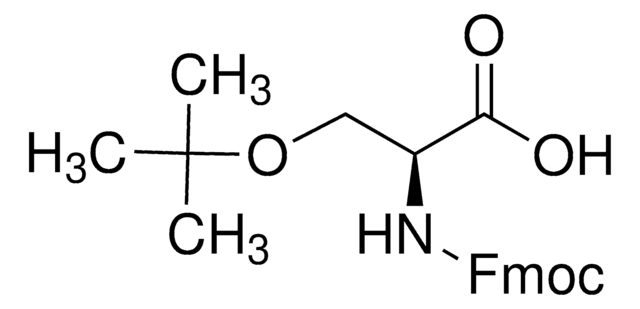
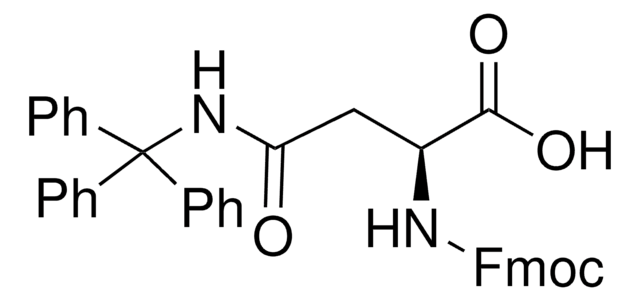
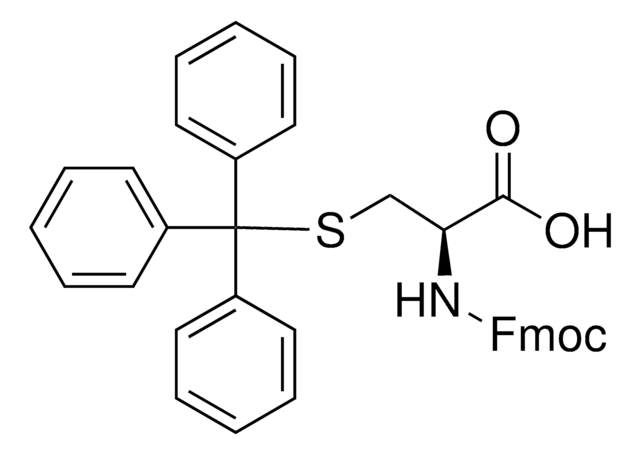
추천 제품
Quality Level
분석
≥97.0%
광학 활성
[α]20/D −25±2°, c = 1% in DMF
반응 적합성
reaction type: C-H Activation
reaction type: Fmoc solid-phase peptide synthesis
reagent type: ligand
reaction type: Peptide Synthesis
mp
152-156 °C (lit.)
152-156 °C
응용 분야
peptide synthesis
작용기
Fmoc
amine
carboxylic acid
저장 온도
2-8°C
SMILES string
CC(C)C[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C21H23NO4/c1-13(2)11-19(20(23)24)22-21(25)26-12-18-16-9-5-3-7-14(16)15-8-4-6-10-17(15)18/h3-10,13,18-19H,11-12H2,1-2H3,(H,22,25)(H,23,24)/t19-/m0/s1
InChI key
CBPJQFCAFFNICX-IBGZPJMESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Fmoc-Leu-OH can be used as a reactant to synthesize:
- Various oligopeptides by reacting with functionalized α-amino acid hydrochloride salts.
- A cyclic depsipeptide sansalvamide A, a natural product found in marine fungus.
- Streptocidin A−D, decapeptide antibiotics naturally found in Streptomyces sp. Tü 6071.
- Coumaroyl dipeptide amide that can be used for cosmetic applications.
생화학적/생리학적 작용
PPARγ ligand that induces insulin sensitization, but not adipogenesis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Facile solid-phase synthesis of cyclic decapeptide antibiotic streptocidins A-D
Qin C, et al.
Tetrahedron Letters, 45(1), 217-220 (2004)
Markus Beck Erlach et al.
Journal of biomolecular NMR, 69(2), 53-67 (2017-09-16)
For evaluating the pressure responses of folded as well as intrinsically unfolded proteins detectable by NMR spectroscopy the availability of data from well-defined model systems is indispensable. In this work we report the pressure dependence of
Rapid, high-yield, solid-phase synthesis of the antitumor antibiotic sansalvamide A using a side-chain-tethered phenylalanine building block
Lee Y and Silverman RB
Organic Letters, 2(23), 3743-3746 (2000)
Catalytic Oligopeptide Synthesis
Liu Z, et al.
Organic Letters, 20(3), 612-615 (2018)
G Cheng et al.
Langmuir : the ACS journal of surfaces and colloids, 26(7), 4990-4998 (2010-01-16)
The self-assembly and hydrogelation properties of two Fmoc-tripeptides [Fmoc = N-(fluorenyl-9-methoxycarbonyl)] are investigated, in borate buffer and other basic solutions. A remarkable difference in self-assembly properties is observed comparing Fmoc-VLK(Boc) with Fmoc-K(Boc)LV, both containing K protected by N(epsilon)-tert-butyloxycarbonate (Boc). In
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.