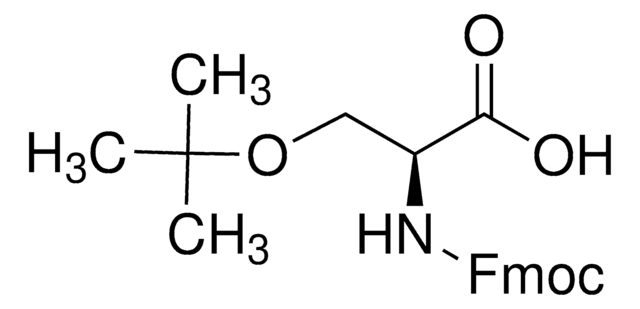
추천 제품
제품명
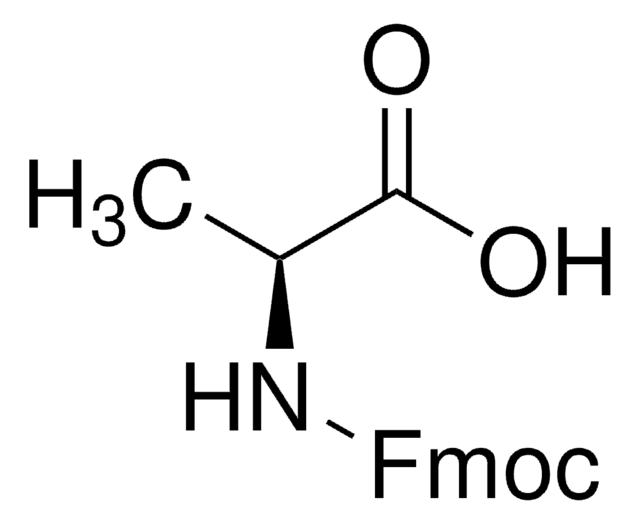
Fmoc-Thr(tBu)-OH, ≥98.0% (HPLC)
Quality Level
분석
≥98.0% (HPLC)
광학 활성
[α]20/D +16±1°, c = 1% in ethyl acetate
반응 적합성
reaction type: C-H Activation
reaction type: Fmoc solid-phase peptide synthesis
reagent type: ligand
reaction type: Peptide Synthesis
응용 분야
peptide synthesis
작용기
Fmoc
amine
carboxylic acid
저장 온도
2-8°C
SMILES string
C[C@@H](OC(C)(C)C)[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C23H27NO5/c1-14(29-23(2,3)4)20(21(25)26)24-22(27)28-13-19-17-11-7-5-9-15(17)16-10-6-8-12-18(16)19/h5-12,14,19-20H,13H2,1-4H3,(H,24,27)(H,25,26)/t14-,20+/m1/s1
InChI key
LZOLWEQBVPVDPR-VLIAUNLRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Fmoc-Thr(tBu)-OH also known as Fmoc-O-tert-butyl-L-threonine, is commonly used as amino acid building block in peptide synthesis.
애플리케이션
Fmoc-Thr(tBu)-OH can be used to synthesize chlorofusin analogues via solid phase peptide synthesis. Additionally, it can serve as a protecting group for both the amine and the hydroxyl functions in solid-phase synthesis of complex depsipeptides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
General Fmoc-Based Solid-Phase Synthesis of Complex Depsipeptides Circumventing Problematic Fmoc Removal
A Lobo-Ruiz
European Journal of Organic Chemistry, 2020, 183-192 (2020)
Solid-phase synthesis of chlorofusin analogues
ECY Woon
The Journal of Organic Chemistry, 72, 5146-5151 (2007)
Gizella Csire et al.
Journal of inorganic biochemistry, 203, 110927-110927 (2019-12-07)
Interaction of copper(II) and nickel(II) ions with the Ac-PHAAAGTHSMKHM-NH2 tridecapeptide containing the His85, His96 and His111 binding sites of human prion protein has been studied by various techniques. pH-potentiometry, UV-Vis and circular dichroism spectroscopy were applied to study the stoichiometry
Zdenek Kukacka et al.
ChemMedChem, 13(9), 909-915 (2018-02-24)
α-Galactosidase (αGal) is a lysosomal enzyme that hydrolyses the terminal α-galactosyl moiety from glycosphingolipids. Mutations in the encoding genes for αGal lead to defective or misfolded enzyme, which results in substrate accumulation and subsequent organ dysfunction. The metabolic disease caused
Stefan Eissler et al.
Journal of peptide science : an official publication of the European Peptide Society, 23(10), 757-762 (2017-06-22)
In solid-phase peptide synthesis, the nominal batch size is calculated using the starting resin substitution and the mass of the starting resin. The starting resin substitution constitutes the basis for the calculation of a whole set of important process parameters
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.