05512
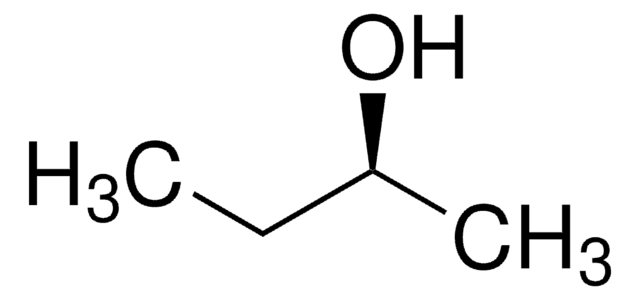
(S)-(−)-1-Phenylethanol
≥98.5% (sum of enantiomers, GC)
동의어(들):
(−)-Methyl phenyl carbinol, (S)-(−)-α-Methylbenzyl alcohol, (S)-(−)-sec-Phenylethyl alcohol
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C8H10O
CAS Number:
Molecular Weight:
122.16
Beilstein:
2039797
MDL number:
UNSPSC 코드:
12352002
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥98.5% (sum of enantiomers, GC)
형태
liquid
광학 활성
[α]/D −45±2°, c = 5% in methanol
광학 순도
enantiomeric ratio: ≥97:3 (GC)
refractive index
n20/D 1.527
bp
88-89 °C/10 mmHg (lit.)
mp
9-11 °C (lit.)
density
1.012 g/mL at 20 °C (lit.)
작용기
hydroxyl
phenyl
SMILES string
C[C@H](O)c1ccccc1
InChI
1S/C8H10O/c1-7(9)8-5-3-2-4-6-8/h2-7,9H,1H3/t7-/m0/s1
InChI key
WAPNOHKVXSQRPX-ZETCQYMHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(S)-(-)-1-Phenylethanol can be prepared from acetophenone via enantioselective bioreduction in the presence of Rhizopus arrhizus as a biocatalyst.
애플리케이션
(S)-(-)-1-Phenylethanol can be used as:
- A starting material to prepare (1S,3R,4S)-1-methyl-3,4-diphenyl-3,4-dihydro-1H-isochromene-3,4-diol (a cyclic hemiacetal) by reacting with benzil via dilithiation reaction.
- A chiral solvent in the symmetric synthesis of substituted spiroundecenetriones via amino acid-catalyzed domino Knoevenagel/Diels-Alder reactions.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Laboratory scale-up synthesis of chiral carbinols using Rhizopus arrhizus
Salvi, NA and Chattopadhyay S
Tetrahedron Asymmetry, 27(4-5), 188-192 (2016)
Synthesis of (1S, 3R, 4S)-1-methyl-3, 4-diphenyl-3, 4-dihydro-1H-isochromene-3, 4-diol
Shishkina IN, et al.
Mendeleev Communications, 6(23), 350-351 (2013)
Organocatalytic Asymmetric Domino Knoevenagel/Diels-Alder Reactions: A Bioorganic Approach to the Diastereospecific and Enantioselective Construction of Highly Substituted Spiro [5, 5] undecane-1, 5, 9-triones
Ramachary DB, et al.
Angewandte Chemie (International Edition in English), 42(35), 4233-4237 (2003)
문서
Chiral Alcohols
Chromatograms
suitable for GC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.