모든 사진(1)
About This Item
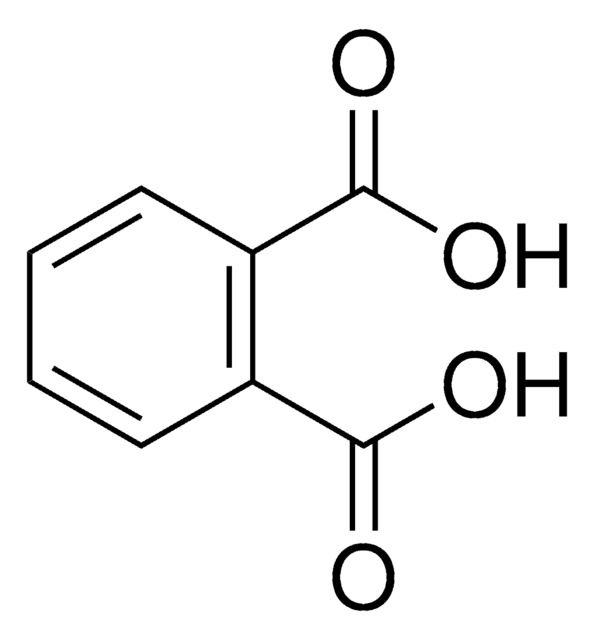
Linear Formula:
C6H4-1,2-(CO2H)2
CAS Number:
Molecular Weight:
166.13
Beilstein:
608199
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.21
추천 제품
Grade
ACS reagent
Quality Level
분석
≥99.5%
양식
powder or crystals
불순물
≤0.05% insolubles
≤0.5% water
무기 잔류물
≤0.02%
mp
210-211 °C (dec.) (lit.)
음이온 미량물
chloride (Cl-): ≤0.001%
nitrate (NO3-): ≤0.005%
sulfate (SO42-): ≤0.005%
양이온 미량물
Fe: ≤0.001%
heavy metals: ≤0.001%
작용기
carboxylic acid
SMILES string
OC(C1=C(C(O)=O)C=CC=C1)=O
InChI
1S/C8H6O4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H,(H,9,10)(H,11,12)
InChI key
XNGIFLGASWRNHJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Phthalic acid (PA, PTA), also called as 1,2-benzenedicarboxylic acid is an aromatic carboxylic acid. It is the ortho form of the three phthalic acid isomers, the other two being isophthalic acid (meta form) and terephthalic acid (para form). PA is the starting material in the synthesis of plasticizers. A study on toxicity reveals that PA shows in vitro and in vivo toxicity. Its mineralization by photocatalysis using TiO2/UV system has been studied. It also is used as a potential tyrosinase inhibitor.
애플리케이션
Phthalic acid may be used as a catalyst for the thiocyanation of aromatics and heteroaromatics and as an interfacial protector for the preparation of ordered mesoporous γ-alumina. It may be used as a model compound in understanding the adsorption of aromatic carboxylic acids on gold surfaces.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
334.4 °F
Flash Point (°C)
168 °C
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Inhibitory effect of phthalic Acid on tyrosinase: the mixed-type inhibition and docking simulations.
Shang-Jun Yin et al.
Enzyme research, 2011, 294724-294724 (2011-06-04)
Tyrosinase inhibition studies are needed due to the medicinal applications such as hyperpigmentation. For probing effective inhibitors of tyrosinase, a combination of computational prediction and enzymatic assay via kinetics was important. We predicted the 3D structure of tyrosinase, used a
A new synthetic procedure for ordered mesoporous γ-alumina using phthalic acid as an interfacial protector.
Huang F, et al.
Materials Letters, 65(2), 244-246 (2011)
Modeling of photocatalytic mineralization of phthalic acid in TiO2 suspension using response surface methodology (RSM).
Abaamrane A, et al.
Desalination and Water Treatment, 53(1), 249-256 (2015)
Phthalic acid as a di-functional organocatalyst for the regioselective thiocyanation of aromatic compounds.
Sajjadifar S, et al.
Scientia Iranica. Transaction C, Chemistry, Chemical Engineering, 21(6), 2005-2005 (2014)
Du Yeon Bang et al.
Toxicological research, 27(4), 191-203 (2011-12-01)
There has been growing concern about the toxicity of phthalate esters. Phthalate esters are being used widely for the production of perfume, nail varnish, hairsprays and other personal/cosmetic uses. Recently, exposure to phthalates has been assessed by analyzing urine for
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.