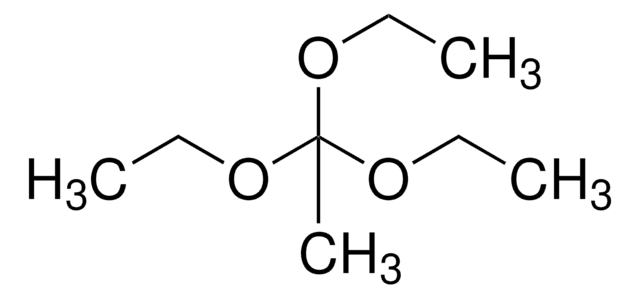
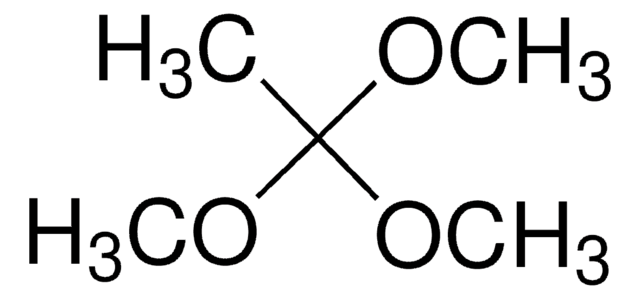
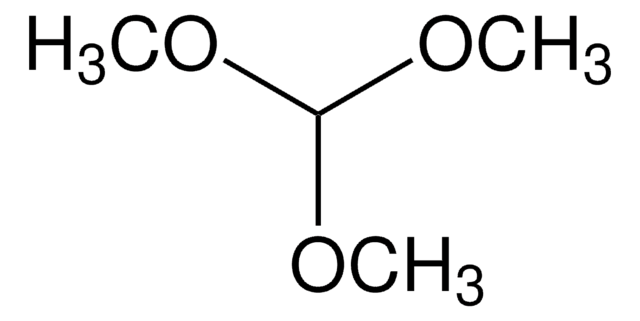
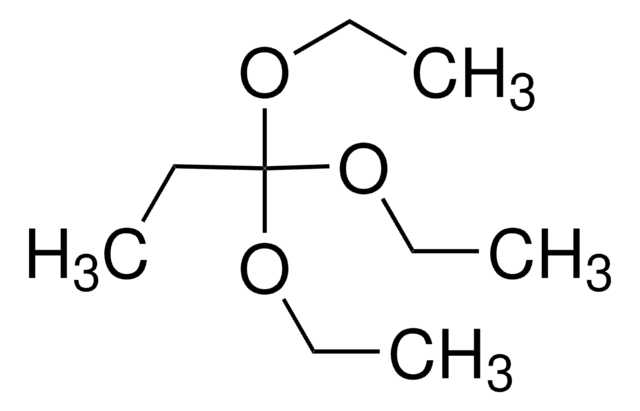
추천 제품
애플리케이션
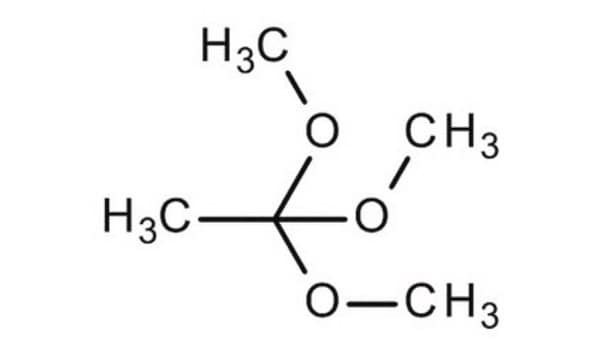
Triethyl orthoacetate is a general reagent used to functionalize alcohols with acetate groups. It can be used in following reactions:
- Stereocontrolled total synthesis of a naturally occuring indole alkaloid, (−)-aspidophytine.
- Conversion of allylic alcohols to γ,δ-unsaturated esters under mild acidic condition, a reaction popularly known as Johnson–Claisen rearrangement.
- Synthesis of heterocycles such as 2-oxazolines and quinazolin-4(3H)-one derivatives.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
102.2 °F - Non-equilibrium method
Flash Point (°C)
39 °C - Non-equilibrium method
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Clay catalysis: condensation of orthoesters with O-substituted aminoaromatics into heterocycles.
Villemin D, et al.
Synthetic Communications, 26(15), 2895-2899 (1996)
An efficient and versatile method for the synthesis of optically active 2-oxazolines: an acid-catalyzed condensation of ortho esters with amino alcohols.
Kamata K, et al.
The Journal of Organic Chemistry, 63(9), 113-3116 (1998)
Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene.
Johnson WS, et al.
Journal of the American Chemical Society, 92(3), 741-743 (1970)
A new approach to the facile synthesis of mono-and disubstituted quinazolin-4 (3H)-ones under solvent-free conditions.
Salehi P, et al.
Tetrahedron Letters, 46(41), 7051-7053 (2005)
Reaction of orthoesters with alcohols in the presence of acidic catalysts: A study.
Kumar, HM et al.
Indian J. Chem. B, 44B(8), 1686-1692 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.