PHR1199
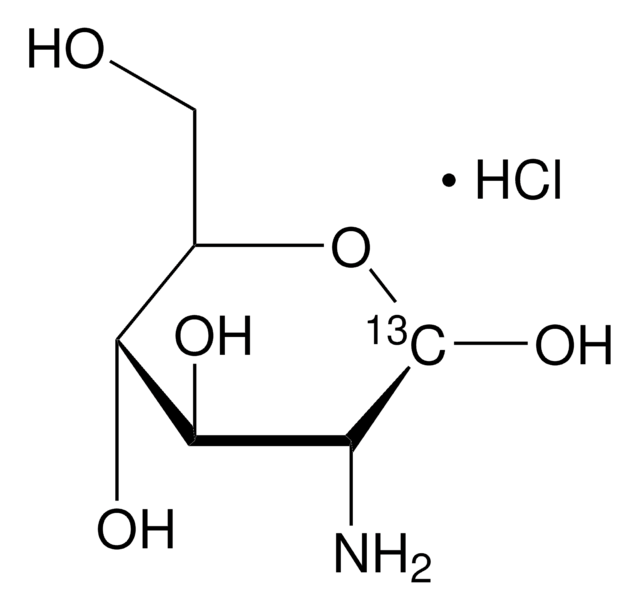
Glucosamine hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
D-(+)-Glucosamine hydrochloride, GLH, 2-Amino-2-deoxy-D-glucose hydrochloride, Chitosamine hydrochloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C6H13NO5 · HCl
CAS Number:
Molecular Weight:
215.63
Beilstein:
4157370
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. Y0001406
traceable to USP 1294207
API family
glucosamine
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
190-194 °C (dec.) (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
Cl.N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O
InChI
1S/C6H13NO5.ClH/c7-3-5(10)4(9)2(1-8)12-6(3)11;/h2-6,8-11H,1,7H2;1H/t2-,3-,4-,5-,6?;/m1./s1
InChI key
QKPLRMLTKYXDST-NSEZLWDYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Glucosamine hydrochloride is a drug prescribed for the remediation of degenerative joint diseases in small animals.
Glucosamine hydrochloride is a drug prescribed for the remediation of degenerative joint diseases in small animals.
애플리케이션
Glucosamine hydrochloride may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations and plasma samples by high performance liquid chromatography (HPLC).
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
생화학적/생리학적 작용
Glucosamine, an amino sugar, is the precursor of the hexosamine biosynthetic pathway leading to the formation of UDP-N-acetylglucosamine (UDP-GlcNAc), which is then used for making glycosaminoglycans, proteoglycans, and glycolipids. D-(+)-Glucosamine inhibits the coaggregation of Candida yeast species with the bacterial strain S. salivarius.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAB3705 in the slot below. This is an example certificate only and may not be the lot that you receive.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Development, comparison, and peak validation of a high-performance liquid chromatography method for direct determination of glucosamine hydrochloride in pharmaceutical formulations
Jin P, et al.
Journal of Liquid Chromatography and Related Technologies, 34(20), 2533-2547 (2011)
Determination of the nutraceutical, glucosamine hydrochloride, in raw materials, dosage forms and plasma using pre-column derivatization with ultraviolet HPLC
Liang Z, et al.
Journal of Pharmaceutical and Biomedical Analysis, 20(5), 807-814 (1999)
Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects
Muniyappa R, et al.
Diabetes, 55(11), 3142-3150 (2006)
Oshra Betzer et al.
ACS nano, 8(9), 9274-9285 (2014-08-19)
A critical problem in the development and implementation of stem cell-based therapy is the lack of reliable, noninvasive means to image and trace the cells post-transplantation and evaluate their biodistribution, final fate, and functionality. In this study, we developed a
Ji-Sun Hwang et al.
Metabolism: clinical and experimental, 64(3), 368-379 (2014-12-18)
This study investigated the potential of glucosamine (GlcN) to affect body weight gain and insulin sensitivity in mice normal and at risk for developing diabetes. Male C57BL/6J mice were fed either chow diet (CD) or a high fat diet (HFD)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.