추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1460736
API family
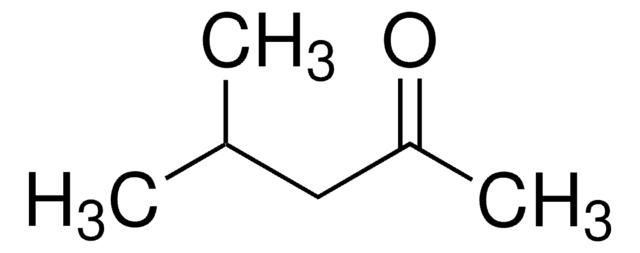
nevirapine
CofA
current certificate can be downloaded
포장
pkg of 50 mg
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
InChI
1S/C12H10N4O/c1-7-4-6-14-11-9(7)15-12(17)8-3-2-5-13-10(8)16-11/h2-6H,1H3,(H,15,17)(H,13,14,16)
InChI key
RKCRKBSFEVQVSX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Nevirapine is found to be the first antiretroviral agent and selective non-competitive inhibitor of the reverse transcriptase, widely used for the treatment of adults and adolescents affected with human immunodeficiency viruses (HIV).
애플리케이션
Nevirapine may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical samples using capillary zone electrophoresis method.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
분석 메모
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAA8554 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Development and validation of a simple and rapid capillary zone electrophoresis method for determination of nnrti nevirapine in pharmaceutical formulations
Filho ZAL, et al.
Journal of the Brazilian Chemical Society, 22(10), 205-2012 (2011)
Simultaneous determination of zidovudine and nevirapine in human plasma by RP-LC
Marchei E, et al.
Journal of Pharmaceutical and Biomedical Analysis, 29(6), 1081-1088 (2002)
Development of a liposomal nanodelivery system for nevirapine
Ramana NL, et al.
Journal of Biomedical Science, 17(1), 57-57 (2010)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.