Y0000520
Nevirapine (anhydrous)
European Pharmacopoeia (EP) Reference Standard
동의어(들):
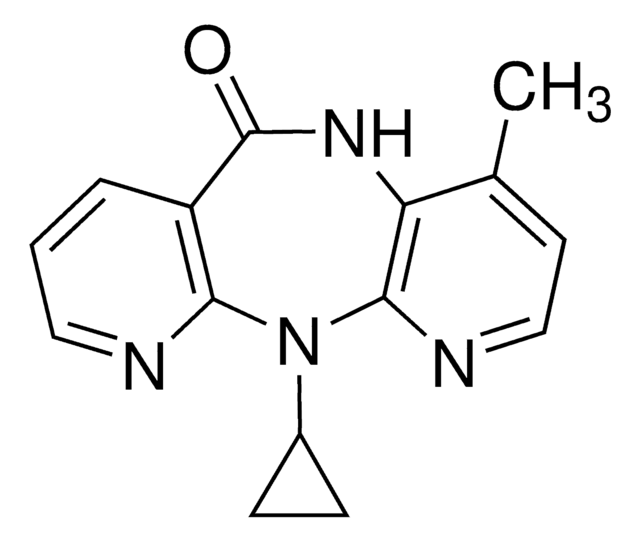
Nevirapine, 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b:2′,3′-e][1,4]diazepin-6-one
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C15H14N4O
CAS Number:
Molecular Weight:
266.30
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
nevirapine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CC1=CC=NC2=C1NC(C(C=CC=N3)=C3N2C4CC4)=O
InChI
1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20)
InChI key
NQDJXKOVJZTUJA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Nevirapine (anhydrous) EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
NNRTI (non-nucleoside reverse transcriptase inhibitor) of HIV-1
Nevirapine is an allosteric, non-nucleoside inhibitor of HIV reverse transcriptase (NNRTI). The Ki for inhibition of wild-type RT by Nevirapine is 200 nM.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Ushma Mehta et al.
The Lancet. Infectious diseases, 7(11), 733-738 (2007-10-27)
The non-nucleoside reverse transcriptase inhibitors (NNRTIs) efavirenz and nevirapine are chemically distinct, but both may cause cutaneous hypersensitivity and hepatotoxicity. We reviewed the literature to assess the evidence for cross-reactivity between nevirapine and efavirenz. All papers, abstracts, or presentations, regardless
Nathan Ford et al.
AIDS (London, England), 27(7), 1135-1143 (2013-01-10)
The risk of adverse drug events associated with nevirapine (NVP) is suggested to be greater in pregnant women. We conducted a systematic review and meta-analysis of severe adverse events in HIV-positive women who initiated NVP while pregnant. We searched six
Ebrahim Bera et al.
South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde, 102(11 Pt 1), 855-859 (2012-11-03)
The package insert for nevirapine (NVP) cautions use in HIV-infected women (including pregnant women) with CD4 counts ≥250 cells/µl. However, recent studies showed that the CD4 count of pregnant women receiving antiretroviral therapy (ART) was not predictive of NVP toxicity.
Laura O Coster et al.
AIDS reviews, 14(2), 132-144 (2012-05-26)
Nevirapine was the first nonnucleoside reverse transcriptase inhibitor that was approved for treatment of HIV infection and quickly became an important component of HAART. As experience with this drug grew, potential toxicities and significant clinical benefits became apparent. With the
M Popovic et al.
Handbook of experimental pharmacology, (196)(196), 437-451 (2009-12-19)
Treatment of HIV-1 infections with nevirapine is associated with skin and liver toxicity. These two organ toxicities range from mild to severe, in rare cases resulting in life-threatening liver failure or toxic epidermal necrolysis. The study of the mechanistic steps
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.