추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1477604
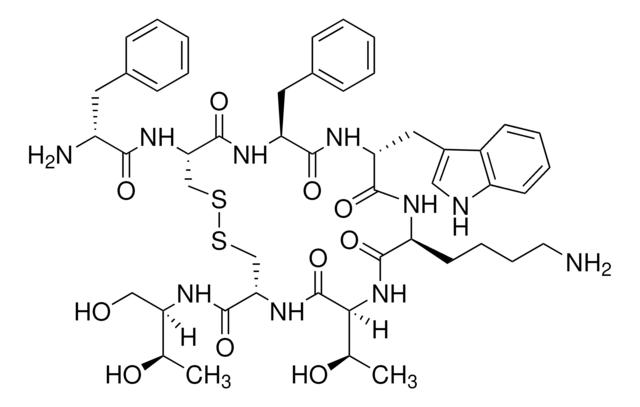
API family
octreotide
형태
powder
CofA
current certificate can be downloaded
포장
pkg of 10 mg
응용 분야
pharmaceutical
저장 온도
-10 to -25°C
InChI
1S/C49H66N10O10S2.2C2H4O2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41;2*1-2(3)4/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64);2*1H3,(H,3,4)/t28-,29?,34?,36?,37?,38?,39-,40?,41?,42?;;/m1../s1
InChI key
QWFYIFWTVZFPRY-AARKYNAGSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Octreotide belongs to the group of synthetic cyclic octapeptides, known for its selectivity towards inhibiting the growth hormone. It binds to the somatostatin receptor 2 (SSTR2), and thereby prevents the secretion pathways of growth hormone. Hence it is used in the treatment of diseases caused by overproduction of growth hormone, such as acromegaly.
애플리케이션
- Octreotide acetate analysis of amino acids including hydrolysis, derivatization of released amino acids with 4-N,N-dimethylaminoazobenzene-4ʹ-sulfonyl chloride (DABS-Cl), and finally their reversed phase-high performance liquid chromatography (RP-HPLC) determination
- Development of a quantitative nuclear magnetic resonance (1H-qNMR) based method for the estimation of octreotide acetate in bulk drug
- Determination of octreotide acetate in pharmaceutical formulations using a stability-indicating capillary zone electrophoresis method (CZE)
- Estimation of octreotide acetate from a peptide-based hydrogel using an ultra-high performance liquid chromatographic method in combination with photo-diode array detection (PDA), following the quality-by-design (QbD) approach
분석 메모
각주
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.