PHR2050
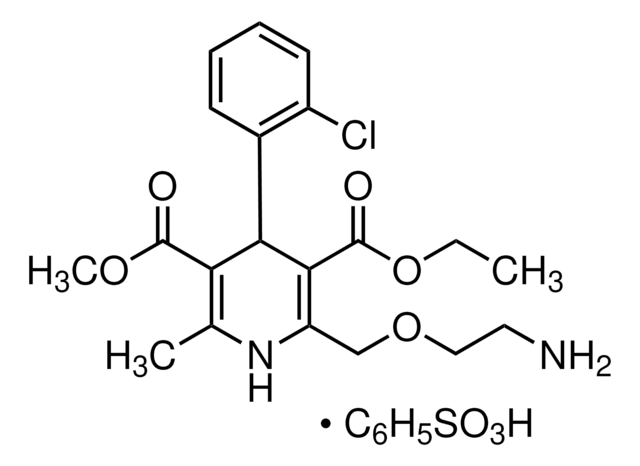
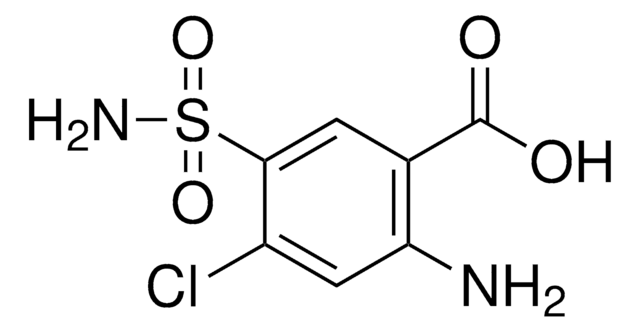
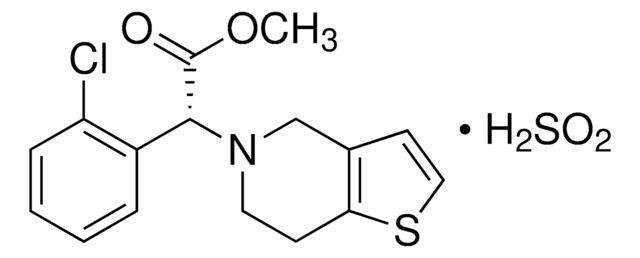
Amlodipine Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
3-Ethyl 5-methyl [2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-3,5-pyridinedicarboxylate] fumarate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H23ClN2O5 · C4H4O4
CAS Number:
Molecular Weight:
522.93
UNSPSC 코드:
41116107
NACRES:
NA.24
추천 제품
일반 설명
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the long-acting calcium channel blocker, amlodipine, that belongs to the class of 1,4-Dihydropyridine (DHP) calcium antagonists. The parent active pharmaceutical ingredient is used in the treatment of hypertension and anginal chest pain.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the long-acting calcium channel blocker, amlodipine, that belongs to the class of 1,4-Dihydropyridine (DHP) calcium antagonists. The parent active pharmaceutical ingredient is used in the treatment of hypertension and anginal chest pain.
애플리케이션
This pharmaceutical secondary standard can also be used as follows:
- Development of a stability-indicating reverse-phase ultra-performance liquid chromatographic (RP-UPLC) method for determining related impurities of S(−)amlodipine and S(−)metoprolol succinate in their combined tablet dosage
- Ultra-high pressure liquid chromatographic (UHPLC) separation and estimation of amlodipine and bisoprolol-related impurities in the combined pharmaceutical formulation of the parent APIs
- Development and validation of a UHPLC method for quantifying impurities of valsartan, amlodipine besylate, and hydrochlorothiazide in their combined tablet dosage
- Separation and determination of amlodipine and atorvastatin, along with their impurities using a stability-indicating RP-HPLC method in their combined solid dosage forms
분석 메모
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
각주
To see an example of a Certificate of Analysis for this material enter LRAB9841 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Stability-indicating method for the determination of assay and quantification of impurities in amlodipine-atorvastatin combination dosage form by RP-HPLC
Sangeetha D and Vadlamudi MK
Journal of Liquid Chromatography and Related Technologies, 40, 576-598 (2017)
Development and Validation of Stability-Indicating RP-UPLC Method for Simultaneous Determination of Related Substances of S (-) Amlodipine and S (-) Metoprolol Succinate in Fixed Dose Combination Tablet Dosage Form
Shitole S, et al.
Chromatography Research International, 2014 (2014)
Robust UHPLC separation method development for multi-API product containing amlodipine and bisoprolol: the impact of column selection
Kormany, R, et al.
Chromatographia, 77, 1119-1127 (2014)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.