추천 제품
Grade
pharmaceutical primary standard
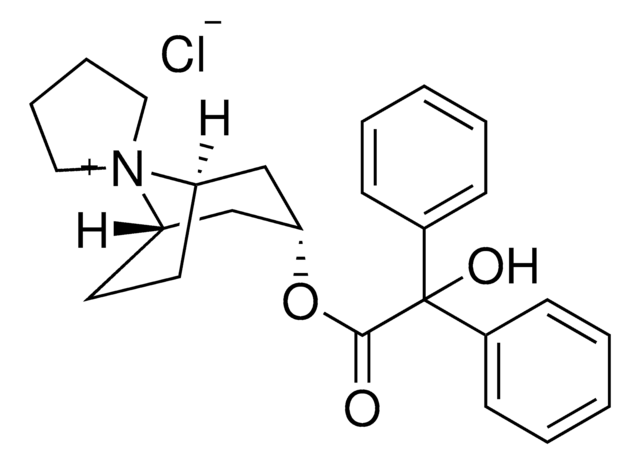
API family
trospium
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
InChI
1S/C25H30NO3.ClH/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26;/h1-6,9-12,21-23,28H,7-8,13-18H2;1H/q+1;/p-1
InChI key
RVCSYOQWLPPAOA-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Trospium chloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
가장 최신 버전 중 하나를 선택하세요:
David A Ginsberg et al.
Neurourology and urodynamics, 30(4), 563-567 (2011-01-27)
Once-daily extended release (XR) trospium chloride, which provides therapeutic trospium plasma concentrations over 24 hours, has demonstrated efficacy in treating overactive bladder (OAB) symptoms as evaluated over a 24-hr period. This analysis examined the effects of trospium XR on diurnal
Peter K Sand et al.
Drugs & aging, 28(2), 151-160 (2011-02-01)
Overactive bladder syndrome (OAB) is associated with various co-morbidities; treatment of these frequently results in multiple medication use (MMU) and the potential for drug-drug interactions, which may lead to adverse events and altered efficacy. With the aging population, the prevalence
Norman R Zinner et al.
Neurourology and urodynamics, 30(7), 1214-1219 (2011-04-05)
Once-daily extended-release (XR) trospium chloride has been evaluated for the treatment of overactive bladder syndrome (OAB) in two 12-week randomized, double-blind, placebo-controlled studies. This pooled analysis of the 9-month open-label extensions to these studies evaluated the long-term efficacy and tolerability
Scott A MacDiarmid et al.
Urology, 77(1), 24-29 (2010-10-26)
This study used pooled data from 2 large, phase III, double-blind, randomized, placebo-controlled studies for a subgroup analysis of the safety and efficacy of trospium chloride extended-release (XR) in men with overactive bladder (OAB). A subgroup analysis was performed on
Mark D Harnett et al.
Clinical drug investigation, 33(2), 133-141 (2012-12-04)
Overactive bladder is a common disorder that affects approximately 34 million adults in the United States. Anticholinergic (antimuscarinic) agents are the most widely used pharmacological option for overactive bladder. This study set out to identify and characterize the influence of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.