Y0000510
Desogestrel for system suitability
European Pharmacopoeia (EP) Reference Standard
동의어(들):
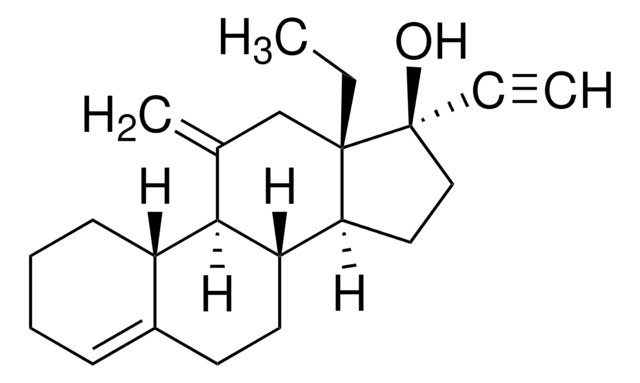
Desogestrel, 13-Ethyl-11-methylene-18,19-dinor-17α-4-pregnen-20-yn-17-ol
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C22H30O
CAS Number:
Molecular Weight:
310.47
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
desogestrel
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CC[C@]12CC(=C)[C@H]3[C@@H](CCC4=CCCC[C@H]34)[C@@H]1CC[C@@]2(O)C#C
InChI
1S/C22H30O/c1-4-21-14-15(3)20-17-9-7-6-8-16(17)10-11-18(20)19(21)12-13-22(21,23)5-2/h2,8,17-20,23H,3-4,6-7,9-14H2,1H3/t17-,18-,19-,20+,21-,22-/m0/s1
InChI key
RPLCPCMSCLEKRS-BPIQYHPVSA-N
유전자 정보
human ... PGR(5241)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Desogestrel for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
D F Archer
American journal of obstetrics and gynecology, 170(5 Pt 2), 1550-1555 (1994-05-01)
Desogestrel is a highly selective gonane progestin. A monophasic formulation containing 150 micrograms of desogestrel and 30 micrograms of ethinyl estradiol has recently been approved as an oral contraceptive (OC) in the United States. Although desogestrel-containing formulations are new to
Giuseppe Benagiano et al.
Annals of the New York Academy of Sciences, 997, 163-173 (2003-12-03)
Progestin-only minipills have been available for over three decades, yet their use has been limited, because of a documented lower efficacy when compared to pills that combine estrogen and progestin. The availability of a new low-dose progestin-only minipill containing 75
P G Stubblefield
American journal of obstetrics and gynecology, 168(3 Pt 2), 1047-1052 (1993-03-01)
Epidemiologic research has shown that current low-dose estrogen oral contraceptives are associated with a low risk of vascular events (e.g., myocardial infarction, stroke, and venous thrombosis or thromboembolism). Yet questions still persist about the effects of low-dose oral contraceptives on
L Laurendeau
Canadian family physician Medecin de famille canadien, 42, 62-71 (1996-01-01)
A review of clinical trials of changes in lipoprotein composition in women receiving oral contraceptives containing desogestrel; a comparison of the trials' findings and a discussion of their clinical significance. Using MEDLINE, we searched for articles published in English and
R T Burkman
American journal of obstetrics and gynecology, 168(3 Pt 2), 1033-1040 (1993-03-01)
Desogestrel is a gonane progestogen that in early studies had an improved ratio between desired progestational effects and undesired androgenic effects. A review of more than 50 clinical studies suggests that desogestrel differs from progestins currently used in oral contraception
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.