추천 제품
Grade
pharmaceutical primary standard
API family
fusidic acid
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
−20°C
SMILES string
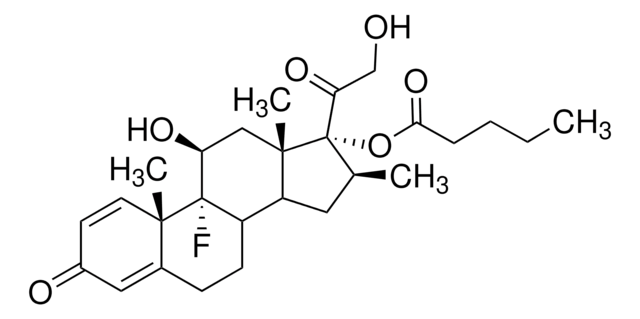
[H][C@@]12CC[C@@]3(C)[C@@]([H])([C@H](O)C[C@@]4([H])\C([C@H](C[C@]34C)OC(C)=O)=C(/CC\C=C(\C)C)C(O)=O)[C@@]1(C)CC[C@@H](O)[C@H]2C
InChI
1S/C31H48O6/c1-17(2)9-8-10-20(28(35)36)26-22-15-24(34)27-29(5)13-12-23(33)18(3)21(29)11-14-30(27,6)31(22,7)16-25(26)37-19(4)32/h9,18,21-25,27,33-34H,8,10-16H2,1-7H3,(H,35,36)/b26-20-/t18-,21-,22-,23+,24+,25-,27-,29-,30-,31-/m0/s1
InChI key
IECPWNUMDGFDKC-MZJAQBGESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Fusidic acid EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Suppresses nitric oxide lysis of pancreatic islet cells. Inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
J D Wilkinson
The British journal of dermatology, 139 Suppl 53, 37-40 (1999-02-17)
Fusidic acid is an antibiotic that belongs to a group of its own, the fusidanes. The molecule has a steroid-like structure but does not possess any steroid activity. The structure is thought to be responsible for the steroid-like high penetration
Benjamin P Howden et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 42(3), 394-400 (2006-01-05)
Fusidic acid has activity against a range of pathogens but has mainly been used to treat staphylococcal infections. Fusidic acid monotherapy, especially topical preparations, has been strongly associated with the emergence of fusidic acid resistance among both methicillin-resistant Staphylococcus aureus
J Turnidge et al.
International journal of antimicrobial agents, 12 Suppl 2, S35-S44 (1999-10-21)
Resistance to fusidic acid is determined by a number of mechanisms. The best described are alterations in elongation factor G, which appear in natural mutants that are harboured at low rates in normal populations of staphylococci (10(6) to 10(8)). Altered
K Christiansen
International journal of antimicrobial agents, 12 Suppl 2, S3-S9 (1999-10-21)
Unlike trials conducted today on new antimicrobials, the introduction of fusidic acid was not accompanied by extensive studies on toxicity and side effects. The early studies on small numbers of patients reported fusidic acid to be a nontoxic drug with
Helmut Schöfer et al.
European journal of dermatology : EJD, 20(1), 6-15 (2009-12-17)
Studies on the clinical efficacy of fusidic acid in skin and soft-tissue infections (SSTIs), notably those due to Staphylococcus aureus, are reviewed. Oral fusidic acid (tablets dosed at 250 mg twice daily, or a suspension for paediatric use at 20
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.