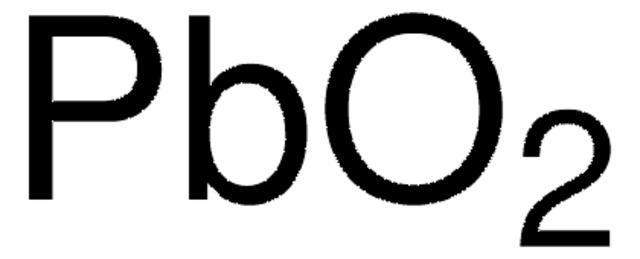추천 제품
vapor pressure
10 mmHg ( 0 °C)
Quality Level
grade
puriss. p.a.
분석
≥99.0% (KT)
형태
powder
불순물
≤0.001% total nitrogen (N)
색상
yellow
pH
9.9 (20 °C, 100 g/L)
mp
886 °C (lit.)
음이온 미량물
chloride (Cl-): ≤20 mg/kg
양이온 미량물
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤10 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Zn: ≤5 mg/kg
SMILES string
O=[PbH2]
InChI
1S/O.Pb
InChI key
YEXPOXQUZXUXJW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Lead(II) oxide is a crystalline solid, which can be prepared by decomposition of lead carbonate or by heating molten lead in the presence of air. It can be used in the synthesis of lead(II) ethanoate by reaction with ethanoic acid.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Lact. - Repr. 1A - STOT RE 1
표적 기관
Central nervous system,Kidney,Blood
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.