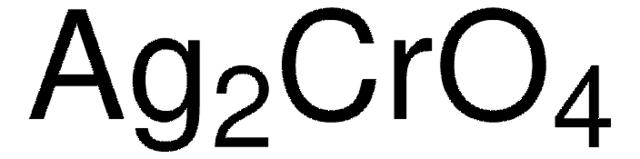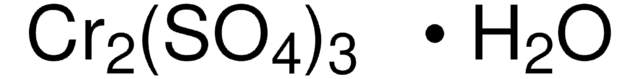모든 사진(3)
About This Item
실험식(Hill 표기법):
CrO3
CAS Number:
Molecular Weight:
99.99
EC Number:
MDL number:
UNSPSC 코드:
12352303
PubChem Substance ID:
NACRES:
NB.24
분석:
≥98.0%
Grade:
ACS reagent
양식:
powder or crystals
추천 제품
Grade
ACS reagent
Quality Level
분석
≥98.0%
양식
powder or crystals
반응 적합성
reagent type: oxidant
불순물
≤0.01% insolubles
pH
<1 (20 °C, 100 g/L)
mp
196 °C (dec.) (lit.)
음이온 미량물
chloride (Cl-): ≤0.005%
nitrate (NO3-): ≤0.05%
sulfate (SO42-): ≤0.005%
양이온 미량물
Fe, Al, Ba: ≤0.03%
Na: ≤0.2%
SMILES string
O=[Cr](=O)=O
InChI
1S/Cr.3O
InChI key
WGLPBDUCMAPZCE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chromium(VI) oxide is an oxidizing reagent for the oxidation of carbon-hydrogen bonds to alcohols, alkylaromatics to ketones and carboxylic acids, alkenes to α, β-unsaturated ketones, arenes to quinones, and alcohols to aldehydes, ketones, acids, and keto acids.
신호어
Danger
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Eye Dam. 1 - Muta. 1B - Ox. Sol. 1 - Repr. 2 - Resp. Sens. 1 - Skin Corr. 1A - Skin Sens. 1 - STOT RE 1 Inhalation - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Chromium (VI) Oxide
Freeman F, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Chromium (VI) Oxide.
Freeman F, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2008)
Chromium (VI) oxide-catalyzed oxidation of arenes with periodic acid.
Yamazaki S.
Tetrahedron Letters, 42(19), 3355-3357 (2001)
L Vayssieres et al.
Journal of nanoscience and nanotechnology, 1(4), 385-388 (2003-08-14)
We are reporting here on the inexpensive fabrication and optical properties of an iron(III) oxide-chromium(III) oxide nanocomposite thin film of corundum crystal structure. Its novel and unique-designed architecture consists of uniformed, well-defined and oriented nanorods of Hematite (alpha-Fe2O3) of 50
Adam Szelag et al.
Polish journal of pharmacology, 55(6), 1097-1103 (2004-01-20)
Hexavalent chromium compounds exhibit higher toxicity than its trivalent compounds since chromium ions in the +6 oxidation state easily cross biological membranes. It has recently been proposed that substances reducing chromium ions from the +6 to the less toxic +3
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.