추천 제품
Grade
ACS reagent
Quality Level
분석
≥98%
98.0-101.0% (ACS specification)
형태
powder, crystals or chunks
반응 적합성
reagent type: catalyst
core: zinc
불순물
≤0.005% insolubles
pH
6.0-7.0 (25 °C, 5%)
음이온 미량물
chloride (Cl-): ≤5 ppm
sulfate (SO42-): ≤0.005%
양이온 미량물
Ca: ≤0.005%
Fe: ≤5 ppm
K: ≤0.01%
Mg: ≤0.005%
Na: ≤0.05%
Pb: ≤0.002%
SMILES string
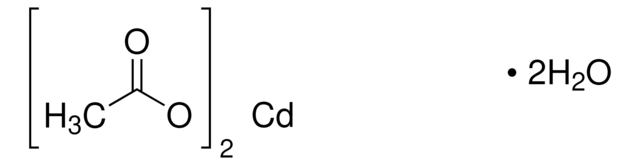
O.O.CC(=O)O[Zn]OC(C)=O
InChI
1S/2C2H4O2.2H2O.Zn/c2*1-2(3)4;;;/h2*1H3,(H,3,4);2*1H2;/q;;;;+2/p-2
InChI key
BEAZKUGSCHFXIQ-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Zinc acetate dehydrate is an organic zinc salt. Its enthalpy of formation has been studied using solution calorimetry. Its vibrational spectra has been analyzed. It decomposes into ZnO (zinc oxide) nanoparticles which has been investigated using time-of-flight secondary ion mass spectrometry (TOF-SIMS). A study on the crystalline structure has revealed that it is monoclinic with space group C2/c.
애플리케이션
Zinc acetate dehydrate was used as seeds to grow ZnO nanorods on the microfibers of PET (polyethylene terephthalate) fabric.
It may be used in the synthesis of layered Zn-arylphosphonates with potential application in sorption, ion exchange or catalysis. Also it may be used in the ultrasonic preparation of zinc sulfide nanoparticles coated on silica particles.
It may be used in the synthesis of layered Zn-arylphosphonates with potential application in sorption, ion exchange or catalysis. Also it may be used in the ultrasonic preparation of zinc sulfide nanoparticles coated on silica particles.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Development of superhydrophilic and superhydrophobic polyester fabric by growing Zinc Oxide nanorods.
Ashraf M, et al.
Journal of Colloid and Interface Science, 394, 545-553 (2013)
The crystal structure of zinc acetate dihydrate, Zn(CH3COO)2.2H2O.
Niekerk V, et al.
Acta Crystallographica, 6(8-9), 720-723 (1953)
Surface synthesis of zinc sulfide nanoparticles on silica microspheres: sonochemical preparation, characterization, and optical properties.
Dhas, N.A. et al.
Chemistry of Materials, 11(3), 806-813 (1999)
Vibrational spectra and structures of zinc carboxylates I. Zinc acetate dihydrate.
Ishioka T, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 54(12), 1827-1835 (1998)
Synthesis and characterization of layered zinc biphenylylenebis (phosphonate) and three mixed-component arylenebis (phosphonate)/phosphates.
Zhang, B. et al.
Inorganic Chemistry, 37(8), 1844-1852 (1998)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.