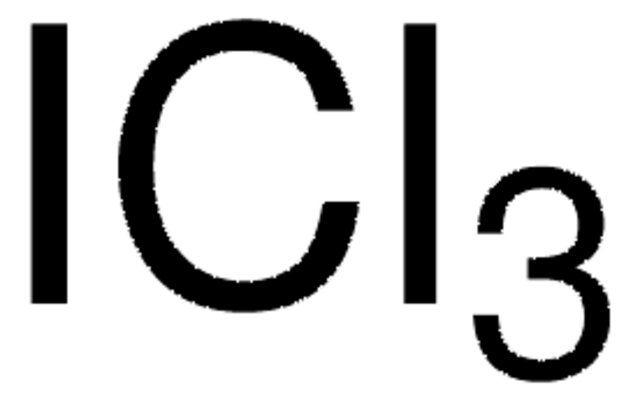추천 제품
Grade
ACS reagent
형태
solid or liquid
불순물
≤0.005% insolubles
bp
97.4 °C (lit.)
solubility
acetic acid: soluble(lit.)
acetone: soluble(lit.)
alcohol: soluble(lit.)
carbon disulfide: soluble(lit.)
density
3.24 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
ClI
InChI
1S/ClI/c1-2
InChI key
QZRGKCOWNLSUDK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Iodine monochloride is an interhalogen compound. It forms various complexes with ethyl, isopropyl and t-butylbenzenes. Equilibrium constants for the formation of these complexes have been evaluated. It affords electrically conducting solution on dissolution in polar solvents. Its reaction with thymidine, 3-mono- and 3,3′,5′-trialkylsubstitued thymidine showed that it helps in deglycosylation, anomerization and isomerization of thymidine.
애플리케이션
Iodine monochloride (ICl) may be employed for the halogenation of methoxy and dimethoxybenzenes. It may be used for the synthesis of flavones.
Iodine monochloride may be used in the synthesis of the following:
- 2-(4-haloisoquinolin-1-yl)ethanol derivatives
- 5-iodosalicylaldehydes
- 5-aryl-6-iodo-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines
- (±)-1-cyclohexyl-4-iodo-3-methoxybutan-1-ol
- (±)-4-Iodo-3-methoxy-1-phenylbutan-1-ol
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Synthesis of Luminescent Ethynyl-Extended Regioisomers of Borate Complexes Based on 2-(2'-Hydroxyphenyl) benzoxazole.
23 Massue J, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 19(17), 5375-5386 (2013)
Synthesis and Electrophilic Substitutions of Novel Pyrazolo [1, 5-c]-1, 2, 4-triazolo [4, 3-a] pyrimidines.
Atta KFM.
Molecules (Basel), 16(8), 7081-7096 (2011)
Brisbois RG, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2013)
Eric Stefan et al.
Tetrahedron, 69(36), 7706-7712 (2013-09-24)
Ether transfer methodology is capable of stereoselectively generating 1,3-diol mono- and diethers in good yield. Surprisingly, allylic and benzylic substrates provide none of the desired products when exposed to previously optimized conditions of iodine monochloride. Herein, second-generation activation conditions for
Three-Component Reactions of 2-Alkynylbenzaldoximes and α, β-Unsaturated Carbonyl Compounds with Bromine or Iodine Monochloride.
Ye S, et al.
Advanced Synthesis & Catalysis, 352(10), 1746-1751 (2010)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.