추천 제품
생물학적 소스
synthetic (organic)
Quality Level
무균
non-sterile
양식
powder
solubility
DMSO: soluble 14 mg/mL at ≤60 °C
H2O: insoluble
배송 상태
ambient
저장 온도
room temp
SMILES string
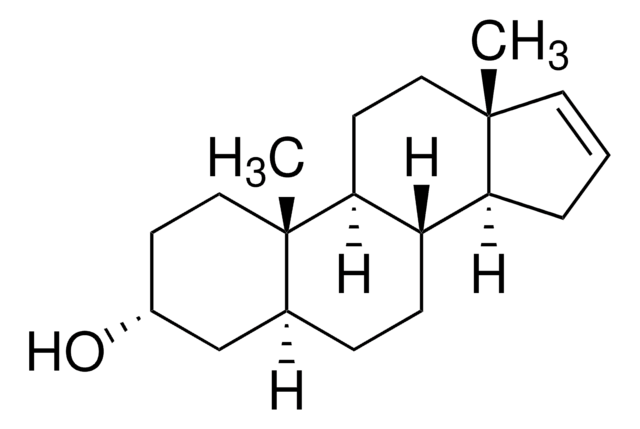
[H][C@@]12CC[C@@]3([H])[C@]4([H])CCC[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@H](O)C2
InChI
1S/C19H32O/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18/h13-17,20H,3-12H2,1-2H3/t13-,14-,15-,16-,17-,18-,19-/m0/s1
InChI key
DJTOLSNIKJIDFF-LOVVWNRFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
5α-Androstan-3β-ol has been used as a cortisone analog to test its effect on the voltage-dependent potassium channel (Kv) current.
생화학적/생리학적 작용
mCAR (constitutive androstane receptor) inverse agonist; testosterone metabolite.
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Andrea Toell et al.
Journal of cellular biochemistry, 85(1), 72-82 (2002-03-14)
Constitutive androstane receptor (CAR) and pregnane X receptor (PXR) are members of the nuclear receptor superfamily that regulate target gene transcription in a ligand-dependent manner. CAR and PXR have a rather broad, overlapping set of ligands that range from natural
Giovanna M Ledda-Columbano et al.
Carcinogenesis, 24(6), 1059-1065 (2003-06-17)
The nuclear receptor Constitutive Androstane Receptor (CAR) binds DNA as a heterodimer with the retinoic-X receptor and activates gene transcription. Previously, in vitro studies have shown that the testosterone metabolites, androstenol and androstenol, inhibit the constitutive transcriptional activity of CAR
A Kassam et al.
The Journal of biological chemistry, 275(6), 4345-4350 (2000-02-08)
The genes encoding the first two enzymes of the peroxisomal beta-oxidation pathway, acyl-CoA oxidase (AOx) and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase (HD), contain upstream cis-acting regulatory regions termed peroxisome proliferator response elements (PPRE). Transcription of these genes is mediated through the binding
Yan Cai et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 15(1), 89-96 (2002-01-23)
Nuclear receptors constitutive androstane receptor (CAR) and pregnane X receptor (PXR) cross talk and serve as xenobiotic sensors to form a safety net against the toxic effects of harmful substances. Retinoid x receptor alpha (RXRalpha) dimerizes with CAR and PXR.
Ping Li et al.
British journal of pharmacology, 158(5), 1322-1329 (2009-08-26)
Potentiating neurosteroids are some of the most efficacious modulators of the mammalian GABA(A) receptor. One of the crucial interactions may be between the C20 ketone group (D-ring substituent at C17) of the neurosteroid, and the N407 and Y410 residues in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.