C8895
Clofazimine
≥98% (TLC), powder, antimycobacterial rimonphenazine
동의어(들):
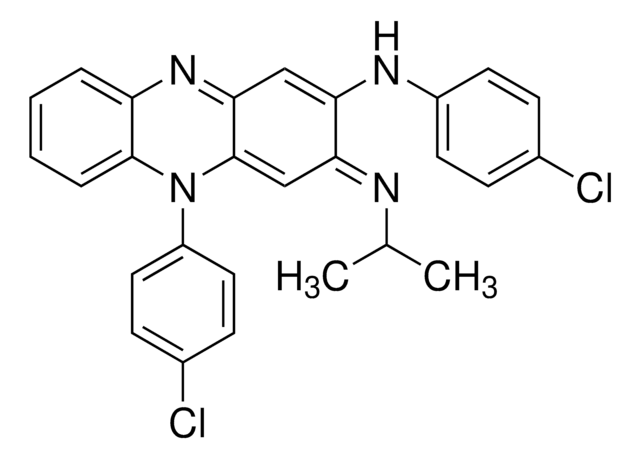
N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(isopropylimino)phenazin-2-amine
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
실험식(Hill 표기법):
C27H22Cl2N4
CAS Number:
Molecular Weight:
473.40
EC Number:
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
제품명
Clofazimine,
양식
powder
Quality Level
항생제 활성 스펙트럼
mycobacteria
동작 모드
protein synthesis | interferes
주관자
Novartis
SMILES string
CC(C)\N=C1/C=C2N(c3ccc(Cl)cc3)c4ccccc4N=C2C=C1Nc5ccc(Cl)cc5
InChI
1S/C27H22Cl2N4/c1-17(2)30-24-16-27-25(15-23(24)31-20-11-7-18(28)8-12-20)32-22-5-3-4-6-26(22)33(27)21-13-9-19(29)10-14-21/h3-17,31H,1-2H3/b30-24+
InChI key
WDQPAMHFFCXSNU-BGABXYSRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Clofazimine is a riminophenazine, which is used as an antileprosy drug. It is used to treat multidrug-resistant tuberculosis. Clofazimine prevents neutrophil motility and lymphocyte transformation. It has anti-inflammatory effect, which is used to treat discoid lupus erythematosus. Clofazimine has immunosuppressive property.
애플리케이션
Clofazimine has been used:
- for antimicrobial preparation
- to study its accumulation on macrophages to form crystal-like drug inclusions (CLDIs)
- to model drug-induced hepatic granulomatous inflammation
- to study the in vivo cargo storage capacity of macrophages
특징 및 장점
This compound was developed by Novartis. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
저장 및 안정성
This product is stored at room temperature.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Macrophage-mediated clofazimine sequestration is accompanied by a shift in host energy metabolism
Trexel J, et al.
Journal of Pharmaceutical Sciences, 106(4), 1162-1174 (2017)
Mode of action of clofazimine and combination therapy with benzothiazinones against Mycobacterium tuberculosis
Lechartier B and Cole ST
Antimicrobial Agents and Chemotherapy, 59(8), 4457-4463 (2015)
The Antimicrobial Drugs (2000)
Sarah Schmidt Grant et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(30), 12147-12152 (2012-07-11)
During Mycobacterium tuberculosis infection, a population of bacteria likely becomes refractory to antibiotic killing in the absence of genotypic resistance, making treatment challenging. We describe an in vitro model capable of yielding a phenotypically antibiotic-tolerant subpopulation of cells, often called
Jacques H Grosset et al.
American journal of respiratory and critical care medicine, 188(5), 608-612 (2013-07-05)
Although observational studies suggest that clofazimine-containing regimens are highly active against drug-resistant tuberculosis, the contribution of clofazimine for the treatment of this disease has never been systematically evaluated. Our goal was to directly compare the activity of a standard second-line
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.