추천 제품
Quality Level
분석
≥97% (HPLC)
solubility
methanol: 1 mg/mL
저장 온도
2-8°C
SMILES string
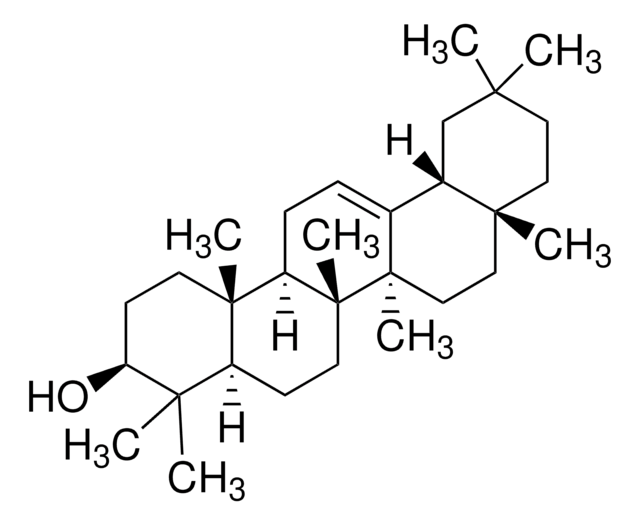
O[C@H]1CC[C@@]2(C)[C@](CC[C@]3(C)[C@]2([H])CC=C4[C@@]3(C)CC[C@]5(C(O)=O)[C@@]4([H])CC(C)(C)CC5)([H])[C@]1(C)CO
InChI
1S/C30H48O4/c1-25(2)13-15-30(24(33)34)16-14-28(5)19(20(30)17-25)7-8-22-26(3)11-10-23(32)27(4,18-31)21(26)9-12-29(22,28)6/h7,20-23,31-32H,8-18H2,1-6H3,(H,33,34)/t20-,21+,22+,23-,26-,27-,28+,29+,30-/m0/s1
InChI key
PGOYMURMZNDHNS-MYPRUECHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Hederagenin (3,23-Dihydroxyolean-12-en-28-acid), a triterpenoid saponin, is used to study the properties of triterpenoid saponins as biopesticides that prevent fungal growth, bacterial growth, and viral plant diseases. Hederagenin may be used as a reference in assays to detect it in biological fluids.
생화학적/생리학적 작용
Saponin. Anti-inflammatory.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Jyoti Kalola et al.
Journal of AOAC International, 91(5), 1174-1178 (2008-11-05)
Fruit pericarp of Sapindus species are reported to contain glycosides with hederagenin as an aglycone. To free the aglycone from the glycosides, they need to be hydrolyzed, and the commonly used method is hydrolysis with either hydrochloric or sulfuric acid.
L Jayasinghe et al.
Phytochemistry, 40(3), 891-897 (1995-10-01)
Seven new saponins, all glycosides of hederagenin (3 beta,23- dihydroxyolean-12-en-28-oic acid), were isolated from the stem of Pometia eximia along with hederagenin and two known saponins. Their structures were established as 28-O-beta-D-apiosyl(1-->2)-beta-D-glucopyranosyl-, 3-O-alpha-L- arabinofuranosyl(1-->3)[alpha-L-rhamnopyranosyl (1-->2)]-beta-D- xylopyranosyl-, 3-O-beta-D-apiosyl(1-->3)[alpha-L-rhamnopyranosyl-(1-->2)-beta-D- glucopyranosyl-, 3-O-alpha-L-arabinofuranosyl(1-->3)[alpha-L-rhamnopyranosyl- (1-->2)]-beta-L-arabinopyranosyl-
Anti-inflammatory activities of hederagenin and crude saponin isolated from Sapindus mukorossi Gaertn.
K Takagi et al.
Chemical & pharmaceutical bulletin, 28(4), 1183-1188 (1980-01-01)
Yao Su et al.
Journal of Asian natural products research, 15(1), 71-77 (2012-10-31)
A novel pyrrolidine alkaloid, (2R*,3S*,5S*)-N,2-dimethyl-3-hydroxy-5-(10-phenyldecyl)pyrrolidine (1), and 17 known compounds were isolated from Arisaema franchetianum Engl. (Araceae) tubers. The 17 compounds were bergenin (2), emodin (3), caffeic acid (4), nobiletin (5), 3-O-β-d-galactopyranosyl-hederagenin 28-O-β-d-xylopyranosyl(1 → 6)-β-d-galactopyranosyl ester (6), coniferin (7), qingyangshengenin (8), methylconiferin
Marina A Naoumkina et al.
The Plant cell, 22(3), 850-866 (2010-03-30)
Saponins, an important group of bioactive plant natural products, are glycosides of triterpenoid or steroidal aglycones (sapogenins). Saponins possess many biological activities, including conferring potential health benefits for humans. However, most of the steps specific for the biosynthesis of triterpene
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.