모든 사진(4)
About This Item
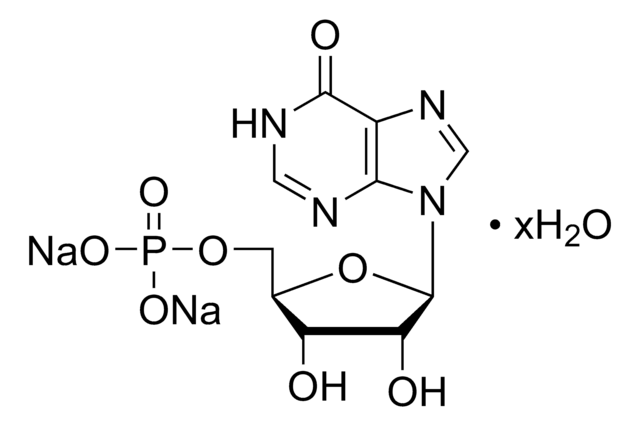
실험식(Hill 표기법):
C10H14N4O11P2
CAS Number:
Molecular Weight:
428.19
MDL number:
UNSPSC 코드:
41106305
PubChem Substance ID:
NACRES:
NA.51
추천 제품
생물학적 소스
microbial
Quality Level
분석
≥96%
형태
powder
solubility
water: 50 mg/mL, clear to very slightly hazy, colorless
저장 온도
−20°C
SMILES string
[Na+].[Na+].[Na+].O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP([O-])(=O)OP([O-])([O-])=O)n2cnc3C(=O)NC=Nc23
InChI
1S/C10H14N4O11P2.3Na/c15-6-4(1-23-27(21,22)25-26(18,19)20)24-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17;;;/h2-4,6-7,10,15-16H,1H2,(H,21,22)(H,11,12,17)(H2,18,19,20);;;/q;3*+1/p-3/t4-,6-,7-,10-;;;/m1.../s1
InChI key
CPIQGMJSIPVOOS-MSQVLRTGSA-K
애플리케이션
Inosine 5′-diphosphate sodium salt has been used for hystoenzymatic demonstration of nucleoside diphosphatase (NDPase).
제조 메모
Prepared from muscle or bacterial ADP.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
STOT SE 2
표적 기관
Eyes,Central nervous system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
John A Duley et al.
Therapeutic drug monitoring, 27(5), 647-654 (2005-09-22)
Metabolism of thiopurine drugs--azathioprine, 6-mercaptopurine, and 6-thioguanine--has provided a powerful pharmacogenetic model incorporating polymorphism of the enzyme thiopurine methyltransferase (TPMT) and the primary active metabolite, thioguanine nucleotide (TGN). However, a sense of uncertainty about the usefulness of TGNs and other
Increased number of microglia in the brain of severe combined immunodeficient (SCID) mice
Lorke DE, et al.
Histochemistry and Cell Biology, 130(4), 693-697 (2008)
Edwin K Jackson et al.
The Journal of pharmacology and experimental therapeutics, 321(2), 799-809 (2007-02-23)
Stimulation of adenylyl cyclase causes cellular efflux of cAMP, and cAMP (unlike adenosine) is stable in blood. Therefore, it is conceivable that cAMP could function as a circulating adenosine prohormone by local target-organ conversion of distally released cAMP to adenosine
Satoko Ohkubo et al.
European journal of pharmacology, 577(1-3), 35-43 (2007-09-20)
In C6 glioma cells, adenine nucleotides, especially AMP, and adenosine inhibited cell proliferation in time- and concentration-dependent manners. alpha,beta-methylene-ADP, an ecto-5'-nucleotidase inhibitor, suppressed the hydrolysis of AMP and reversed the inhibition of cell growth induced by AMP but not by
CD4 microglial expression correlates with spontaneous clinical improvement in the acute Lewis rat EAE model
Almolda B, et al.
Journal of Neuroimmunology, 209(1-2), 65-80 (2009)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.