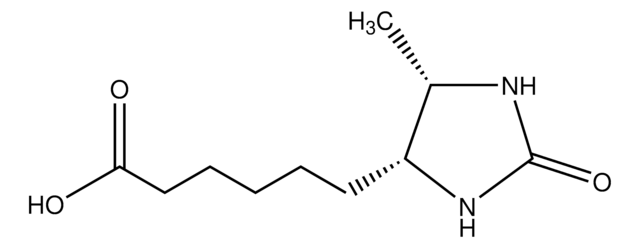
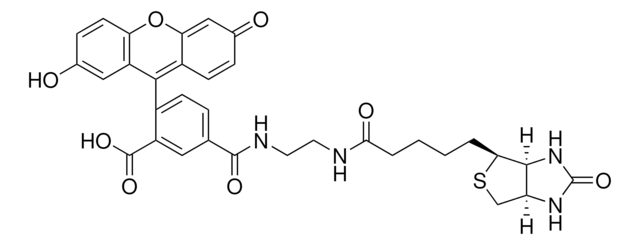
추천 제품
생물학적 소스
synthetic (organic)
Quality Level
분석
≥98% (TLC)
양식
powder
solubility
1 M HCl: 50 mg/mL, clear, colorless
저장 온도
2-8°C
SMILES string
[H][C@]12CS[C@@H](CCCCC(O)=O)[C@@]1([H])NC(=N)N2
InChI
1S/C10H17N3O2S/c11-10-12-6-5-16-7(9(6)13-10)3-1-2-4-8(14)15/h6-7,9H,1-5H2,(H,14,15)(H3,11,12,13)/t6-,7-,9-/m0/s1
InChI key
WWVANQJRLPIHNS-ZKWXMUAHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Iminobiotin is a cyclic guanidino analog of biotin that has high affinity for avidin at high pH (>9) and low affinity at low pH (<6). The proteins that are iminobiotinylated are selectively contained within the avidin column at pH 9 to 11 and may be eluted at pH 4 or by the addition of biotin. This technique may be very useful in the study of cell surface proteins by labeling and selectively recovering the components that are periodate-sensitive from intact human erythrocyte surface. 2-Iminobiotin reversibly inhibits NOS (nitric oxide synthases) via its guanidino group. It inhibits NO biosynthesis and may be important to understand the binding-site interactions for this important class of enzymes. NOS oxidizes the guanidino-nitrogen of L-arginine to produce nitric oxide and L-citrulline.
애플리케이션
2-Iminobiotin has been used as a competitive inhibitor to bind to biotic binding sites on streptavidin in order to restore fluorescence signal.
면책조항
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Hiroyuki Inoue et al.
Langmuir : the ACS journal of surfaces and colloids, 21(18), 8354-8359 (2005-08-24)
Layered thin films composed of avidin and 2-iminobiotin-labeled poly(ethyleneimine) (ib-PEI) were prepared by a layer-by-layer deposition of avidin and ib-PEI on a solid surface, and the disintegration induced by changing environmental pH and adding biotin in the solution was studied.
F Garret-Flaudy et al.
Biotechnology and bioengineering, 71(3), 223-234 (2001-04-06)
Affinity precipitation, especially secondary effect affinity precipitation, has repeatedly been suggested as a valuable technique for the biotechnical downstream process. The present lack of applications is related to the scarcity of predictable affinity macroligands and to the fact that rather
Amesh B Patel et al.
Journal of the American Chemical Society, 126(5), 1318-1319 (2004-02-05)
The strength of a multimolecular system depends on the number of interactions that hold it together. Using dynamic force spectroscopy, we show how the kinetic stability of a system decreases as the number of molecular bonds is increased, as predicted
J Clarkson et al.
Biopolymers, 62(6), 307-314 (2002-02-22)
UV resonance Raman (UVRR) spectroscopy is used to study the binding of biotin and 2-iminobiotin by streptavidin, and the results are compared to those previously obtained from the avidin-biotin complex and new data from the avidin-2-iminobiotin complex. UVRR difference spectroscopy
Cacha Peeters-Scholte et al.
Experimental brain research, 147(2), 200-208 (2002-11-01)
The purpose of this study was to investigate whether combined inhibition of neuronal and inducible nitric oxide synthase (NOS) by 2-iminobiotin, free radical scavenging by allopurinol, and non-protein-bound iron chelation with deferoxamine improved cerebral oxygenation, electrocortical brain activity, and brain
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.