추천 제품
일반 설명
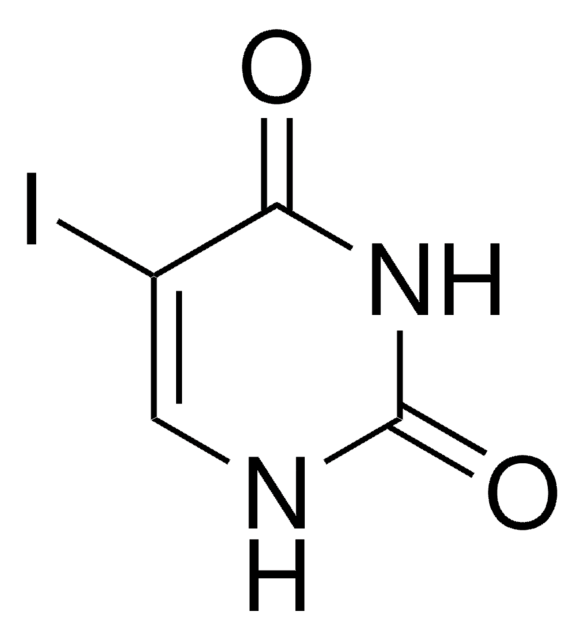
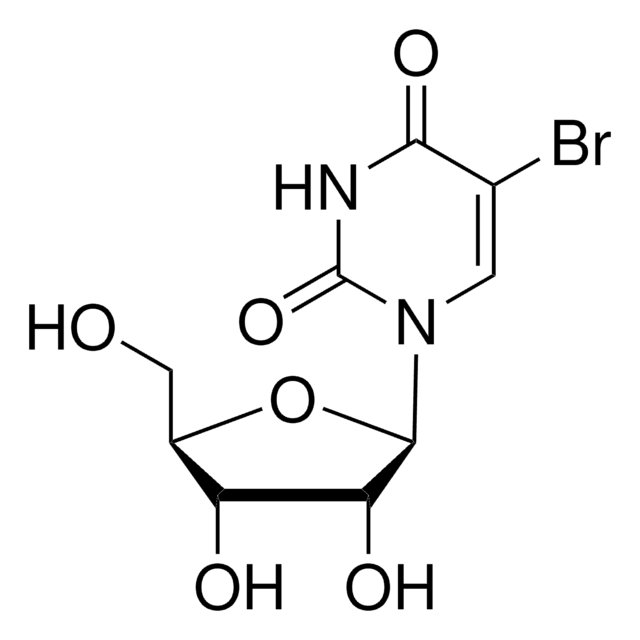
5-Iodocytosine is a modified pyrimidine used in the synthesis of molecules such as pyrrolocytosine and biologically active derivatives.
애플리케이션
5-Iodocytosine has been used as iodinated nucleotide with the single crystals of the 3D DNA designed motif for single anomalous dispersion (SAD) studies.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
T S Heuer et al.
Biochemistry, 36(35), 10655-10665 (1997-09-02)
The virally encoded integrase protein carries out retroviral integration, and to do so, it must make specific interactions with both viral and target DNA sequences. The retroviral integrase has three domains: an amino-terminal region of about 50 amino acids that
R H E Hudson et al.
Nucleosides, nucleotides & nucleic acids, 24(5-7), 581-584 (2005-10-27)
We have employed a tandem Sonogashira/annulation reaction between 5-iodocytosine derivatives and terminal alkynes to yield the fluorescent bicyclic nucleobase pyrrolcytosine. Pyrrolocytosine bearing substituents only on the pyrrole ring are conveniently synthesized from 5-iodocytosine. Water soluble pyrrolocytosines are being investigated as
Chemistry for the synthesis of nucleobase-modified peptide nucleic acid
Hudson RHE, et al.
Pure and Applied Chemistry. Chimie Pure Et Appliquee, 76(7-8), 1591-1598 (2004)
Y Yoshimura et al.
Bioorganic & medicinal chemistry, 8(7), 1545-1558 (2000-09-08)
As part of our ongoing investigation of the synthesis of biologically interesting 2'-modified-4'-thionucleosides, we synthesized 2'-deoxy-2'-fluoro-4'-thioarabinofuranosylpyrimidine and -purine nucleosides, and evaluated their antiviral and antitumor activities. In the pyrimidine series, beta-anomers of 5-ethyluracil, 5-iodouracil, 5-chloroethyluracil, and 5-iodocytosine derivatives showed potent
R H E Hudson et al.
Nucleosides, nucleotides & nucleic acids, 22(5-8), 1029-1033 (2003-10-21)
The Pd0/Cu1 catalyzed cross-coupling of terminal alkynes onto peptide nucleic acid monomers or submonomers bearing iodinated nucleobases has been utilized as a route to base-modified oligomers. Both 5-iodouracil and 5-iodocytosine derivatives undergo the cross-coupling to give the expected products in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.