About This Item
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder or crystals
색상
white to light yellow
mp
151 °C
solubility
ethanol: 25-26 mg/mL, clear, colorless
항생제 활성 스펙트럼
neoplastics
동작 모드
enzyme | inhibits
주관자
Daiichi-Sankyo
저장 온도
2-8°C
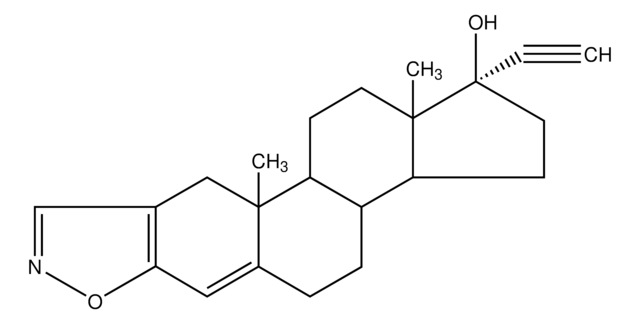
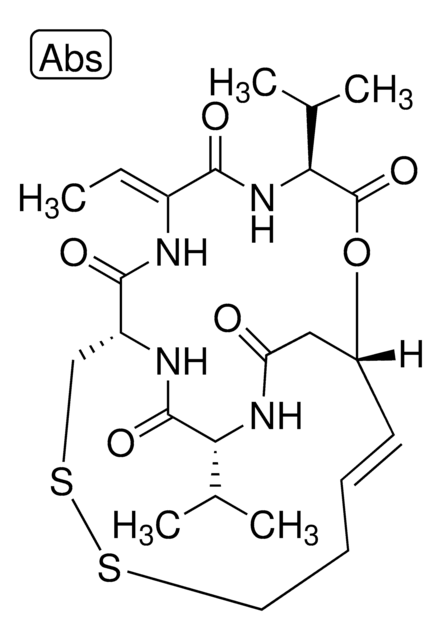
SMILES string
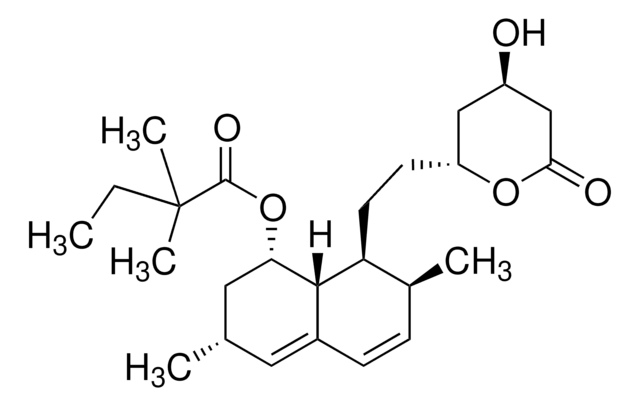
[H][C@]12[C@H](CCC=C1C=C[C@H](C)[C@@H]2CC[C@@H]3C[C@@H](O)CC(=O)O3)OC(=O)[C@@H](C)CC
InChI
1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1
InChI key
AJLFOPYRIVGYMJ-INTXDZFKSA-N
유전자 정보
human ... HMGCL(3155) , HMGCR(3156)
rat ... Hmgcr(25675)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
애플리케이션
- to analyze its effects on chronic lymphocytic leukemia (CLL) cells by cytotoxic assay
- as a prenylation inhibitor to analyze its effects on human embryonic kidney (HEK) cells transfected with K-Ras
- as a statin agent to study its anti-cancer effect on human breast cancer cells and glioblastoma in vitro
생화학적/생리학적 작용
특징 및 장점
포장
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
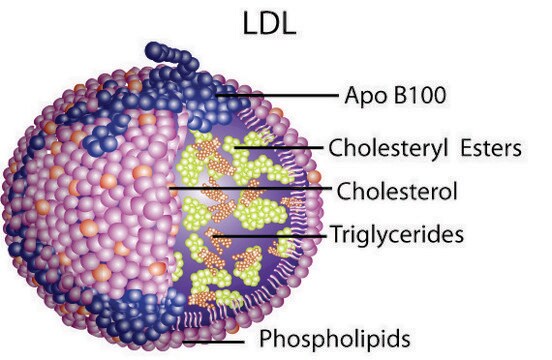
Cholesterol synthesis regulation by dietary levels, LDL receptors control lipid-rich LDL particle transport in cells.
지질 신호전달 연구를 위한 생체 활성 저분자
Discover Bioactive Small Molecules for Lipid Signaling Research
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.