추천 제품
생물학적 소스
synthetic (organic)
Quality Level
설명
zwitterionic
분석
≥98% (perchloric acid titration)
양식
powder
solubility
H2O: 10 mg/mL, clear, colorless
작용기
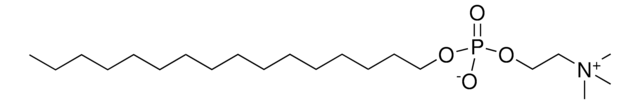
phospholipid
지질 유형
phospholipids
저장 온도
room temp
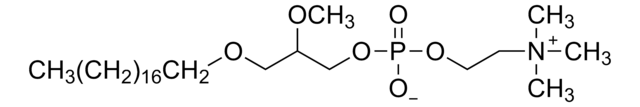
SMILES string
[O-]P(OCC[N+](C)(C)C)(OCCCCCCCCCCCCCCCC)=O
InChI
1S/C21H46NO4P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-27(23,24)26-21-19-22(2,3)4/h5-21H2,1-4H3
InChI key
PQLXHQMOHUQAKB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Easy one-pot synthesis of multifunctionalized indole-pyrrole hybrids as a new class of antileishmanial agents.: This study underlines the necessity for innovative treatments amidst growing resistance to established drugs such as miltefosine which is used as a positive control reference, potentially setting a new direction for antileishmanial drug development (Ciccone et al., 2024).
생화학적/생리학적 작용
Inhibitor of protein kinase C and of phosphatidylcholine synthesis. Used for the treatment of visceral and cutaneous leishmaniasis. Active against metronidazole-resistant and -susceptible strains of Trichomonas vaginalis
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Thomas P C Dorlo et al.
Antimicrobial agents and chemotherapy, 52(8), 2855-2860 (2008-06-04)
The pharmacokinetics of miltefosine in leishmaniasis patients are, to a great extent, unknown. We examined and characterized the pharmacokinetics of miltefosine in a group of patients with Old World (Leishmania major) cutaneous leishmaniasis. Miltefosine plasma concentrations were determined in samples
Thomas P C Dorlo et al.
The Journal of antimicrobial chemotherapy, 67(11), 2576-2597 (2012-07-27)
Miltefosine is an alkylphosphocholine drug with demonstrated activity against various parasite species and cancer cells as well as some pathogenic bacteria and fungi. For 10 years it has been licensed in India for the treatment of visceral leishmaniasis (VL), a
Suresh K Tipparaju et al.
Journal of medicinal chemistry, 51(23), 7344-7347 (2008-11-08)
The first synthesis and biological evaluation of antibiotic 31 (A-33853) and its analogues are reported. Initial screening for inhibition of L. donovani, T. b. rhodesiense, T. cruzi, and P. falciparum cultures followed by determination of IC(50) in L. donovani and
M Rakotomanga et al.
Antimicrobial agents and chemotherapy, 51(4), 1425-1430 (2007-01-24)
Miltefosine (hexadecylphosphocholine [HePC]) is the first orally active antileishmanial drug. Transient HePC treatment of Leishmania donovani promastigotes at 10 microM significantly reduced the phosphatidylcholine content and enhanced the phosphatidylethanolamine (PE) content in parasite membranes, suggesting a partial inactivation of PE-N-methyltransferase.
Maria V Papadopoulou et al.
Journal of medicinal chemistry, 54(23), 8214-8223 (2011-10-26)
A series of novel 2-nitro-1H-imidazole- and 3-nitro-1H-1,2,4-triazole-based aromatic and aliphatic amines were screened for antitrypanosomal activity and mammalian cytotoxicity by the Drugs for Neglected Diseases initiative (DNDi). Out of 42 compounds tested, 18 3-nitro-1,2,4-triazoles and one 2-nitroimidazole displayed significant growth
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.