모든 사진(2)
About This Item
실험식(Hill 표기법):
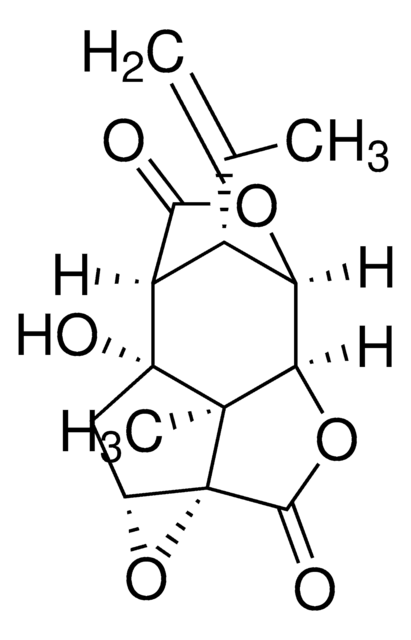
C15H16O6
CAS Number:
Molecular Weight:
292.28
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.77
추천 제품
형태
powder
Quality Level
SMILES string
[H][C@@]12OC(=O)[C@]([H])([C@@H]1C(C)=C)[C@]3(O)C[C@H]4O[C@]45C(=O)O[C@@]2([H])[C@]35C
InChI
1S/C15H16O6/c1-5(2)7-8-11(16)19-9(7)10-13(3)14(8,18)4-6-15(13,21-6)12(17)20-10/h6-10,18H,1,4H2,2-3H3/t6-,7+,8+,9-,10-,13-,14-,15+/m1/s1
InChI key
PIMZUZSSNYHVCU-KBLUICEQSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
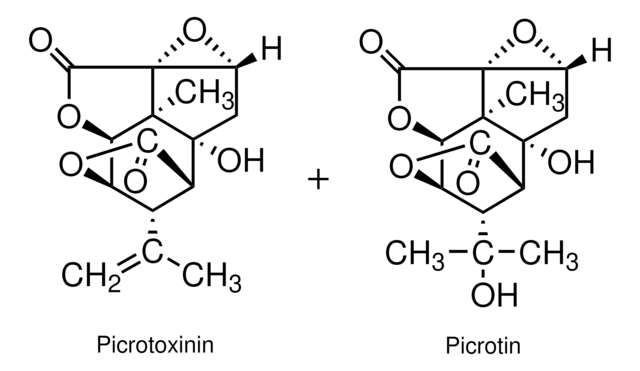
Picrotoxinin is a component of picrotoxin and can be derived from plants. It is a non-nitrogenous compound.
애플리케이션
Picrotoxinin has been used as an agonist of taste 2 receptor member 14 (TAS2R14) to perform an array-based bitter receptor screening assay and to study the effect of calcium buffering and calcium sensor type on its sensitivity.
생화학적/생리학적 작용
Picrotoxinin acts as a potent convulsant.
GABAA receptor antagonist; binds to the GABA receptor-linked Cl− channel.
특징 및 장점
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glycine Receptor page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
기타 정보
Active component of picrotoxin
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
N B Perry et al.
Phytochemical analysis : PCA, 12(1), 69-72 (2001-11-14)
Nuclear Overhauser effect spectroscopy (NOESY) gave full assignments of the 1H-NMR spectra of the picrotoxane neurotoxins tutin, hyenanchin, picrotoxinin and picrotin, as well as the solution conformations of these compounds, consistent with molecular modelling. Fully assigned 13C-NMR data are reported.
Xuebin Chen et al.
Neuropharmacology, 56(1), 318-327 (2008-07-29)
The dihydropyridines (DHPs), nifedipine and nicardipine, modulate native glycine receptors (GlyRs) at micromolar concentrations. Nicardipine has a biphasic potentiating and inhibitory effect, whereas nifedipine causes inhibition only. The present study sought to investigate (1) the molecular mechanism by which these
Eyal Margalit et al.
Visual neuroscience, 28(2), 145-154 (2011-04-06)
Retinal prosthetic devices are being developed to bypass degenerated retinal photoreceptors by directly activating retinal neurons with electrical stimulation. However, the retinal circuitry that is activated by epiretinal stimulation is not well characterized. Whole-cell patch clamp recordings were obtained from
Ping Li et al.
Visual neuroscience, 24(4), 513-521 (2007-07-31)
GABA receptor antagonists produce an unexpectedly significant inhibition of native glycine receptors in retina and in alpha1 or alpha2 homomeric glycine receptors (GlyRs) expressed in HEK 293 cells. In this study we evaluate this phenomenon in heteromeric glycine receptors, formed
Dian-Shi Wang et al.
The Journal of biological chemistry, 282(22), 16016-16035 (2007-04-05)
Contrary to its effect on the gamma-aminobutyric acid type A and C receptors, picrotoxin antagonism of the alpha1 homomeric glycine receptors (GlyRs) has been shown to be non-use-dependent and nonselective between the picrotoxin components picrotoxinin and picrotin. Picrotoxin antagonism of
문서
DISCOVER Bioactive Small Molecules for Neuroscience
신경과학을 위한 생체 활성 저분자
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.