추천 제품
제품명
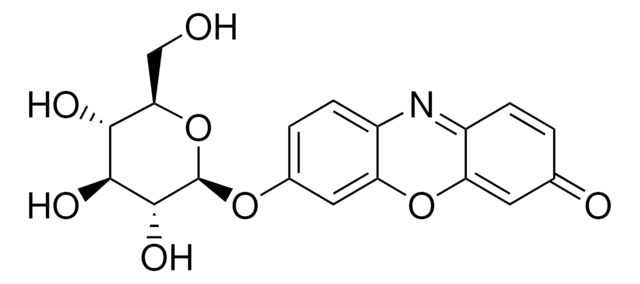
Resorufin β-D-galactopyranoside, ~95%
분석
~95%
Quality Level
양식
powder
solubility
DMSO: 20 mg/mL, clear, orange to red
저장 온도
−20°C
SMILES string
OC[C@H]1O[C@@H](Oc2ccc3N=C4C=CC(=O)C=C4Oc3c2)[C@H](O)[C@@H](O)[C@H]1O
InChI
1S/C18H17NO8/c20-7-14-15(22)16(23)17(24)18(27-14)25-9-2-4-11-13(6-9)26-12-5-8(21)1-3-10(12)19-11/h1-6,14-18,20,22-24H,7H2/t14-,15+,16+,17-,18-/m1/s1
InChI key
QULZFZMEBOATFS-DISONHOPSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
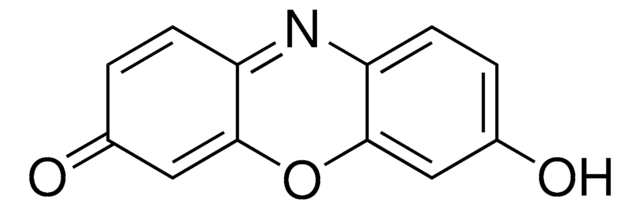
Resorufin β-D-galactopyranoside is a non-fluorescent compound and is orange-yellow in color. It is hydrolyzed by the enzyme β-galactosidase (β-Gal) to yield fluorescent resorufin.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Microchip device for performing enzyme assays
Hadd AG, et al.
Analytical Chemistry, 69(17), 3407-3412 (1997)
Immobilized enzyme kinetics analyzed by flow-through microfluorimetry: resorufin-beta-D-galactopyranoside as a new fluorogenic substrate for beta-galactosidase
Hofmann J and Sernetz M
Analytica Chimica Acta, 163, 67-72 (1984)
Sachin Jambovane et al.
Analytical chemistry, 81(9), 3239-3245 (2009-04-03)
We have demonstrated a multistep enzyme reaction on a chip to determine the key kinetic parameters of enzyme reaction. We designed and fabricated a fully integrated microfluidic chip to have sample metering, mixing, and incubation functionalities. The chip generates a
K D Wittrup et al.
Cytometry, 9(4), 394-404 (1988-07-01)
A novel assay of single-cell exogenous beta-galactosidase activity in Saccharomyces cerevisiae has been developed. Intracellular fluorescence due to the hydrolysis of resorufin-beta-D-galactopyranoside attains a steady state between production of resorufin and its subsequent leakage from the cell. The cells are
Seung-Yong Jung et al.
Langmuir : the ACS journal of surfaces and colloids, 24(9), 4439-4442 (2008-03-26)
A device with femtoliter-scale chambers and controlled reaction initiation was developed for single-molecule enzymology. Initially separated substrate and enzyme streams were rapidly mixed in a microfluidic device and encapsulated in an array of individual microreactors, allowing for enzyme kinetics to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.