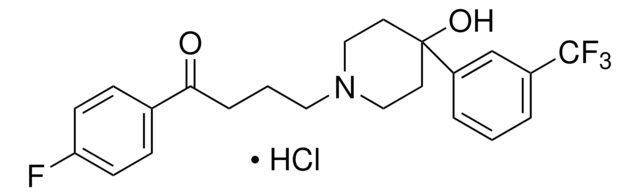
추천 제품
Quality Level
분석
≥98% (HPLC)
형태
solid
색상
light yellow
solubility
DMSO: soluble ~28 mg/mL
H2O: insoluble
주관자
GlaxoSmithKline
저장 온도
2-8°C
SMILES string
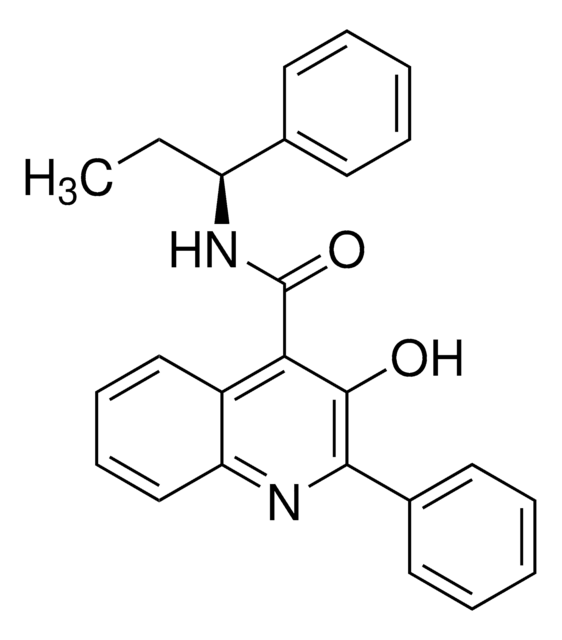
CC[C@H](NC(=O)c1c(C)c(nc2ccccc12)-c3ccccc3)c4ccccc4
InChI
1S/C26H24N2O/c1-3-22(19-12-6-4-7-13-19)28-26(29)24-18(2)25(20-14-8-5-9-15-20)27-23-17-11-10-16-21(23)24/h4-17,22H,3H2,1-2H3,(H,28,29)/t22-/m0/s1
InChI key
MQNYRKWJSMQECI-QFIPXVFZSA-N
유전자 정보
human ... TACR2(6865) , TACR3(6870)
애플리케이션
The pharmacodynamics profile of SB 222200 enables its use as a tool to study physiological and pathophysiological roles of NK-3 receptor in CNS-modulated behaviors.
생화학적/생리학적 작용
SB 222200 is a 2-phenyl-4-quinolinecarboxamides and a selective, reversible and competitive antagonist of human NK-3 receptor that effectively crosses the blood-brain barrier. It inhibits the NK-3 receptor-induced miosis or pupil constriction in conscious rabbits.1,2
Non-peptide NK3 tachykinin receptor antagonist.
특징 및 장점
This compound was developed by GlaxoSmithKline. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
법적 정보
Sold for research purposes under agreement from GlaxoSmithKline
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
A D Medhurst et al.
British journal of pharmacology, 122(3), 469-476 (1997-11-14)
1. Inhibition of NK3 receptor agonist-induced contraction in the rabbit isolated iris sphincter muscle was used to assess the in vitro functional activity of three 2-phenyl-4-quinolinecarboxamides, members of a novel class of potent and selective non-peptide NK3 receptor antagonists. In
H M Sarau et al.
The Journal of pharmacology and experimental therapeutics, 295(1), 373-381 (2000-09-19)
The pharmacological and pharmacokinetic profile of SB-222200 [(S)-(-)-N-(alpha-ethylbenzyl)-3-methyl-2-phenylquinoline-4-car boxami de], a human NK-3 receptor (hNK-3R) antagonist, was determined. SB-222200 inhibited (125)I-[MePhe(7)]neurokinin B (NKB) binding to Chinese hamster ovary (CHO) cell membranes stably expressing the hNK-3 receptor (CHO-hNK-3R) with a K(i)
J García-Ortega et al.
Human reproduction (Oxford, England), 29(12), 2736-2746 (2014-10-16)
Are neurokinin B (NKB), NK3 receptor (NK3R), kisspeptin (KISS1) and kisspeptin receptor (KISS1R) expressed in human ovarian granulosa cells? The NKB/NK3R and kisspeptin/KISS1R systems are co-expressed and functionally active in ovarian granulosa cells. The NKB/NK3R and KISS1/KISS1R systems are essential
Xinhuai Liu et al.
eLife, 10 (2021-01-20)
The necessity and functional significance of neurotransmitter co-transmission remains unclear. The glutamatergic 'KNDy' neurons co-express kisspeptin, neurokinin B (NKB), and dynorphin and exhibit a highly stereotyped synchronized behavior that reads out to the gonadotropin-releasing hormone (GnRH) neuron dendrons to drive
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.