SML2623
(+)-JQ-1 carboxylic acid
≥95% (HPLC)
동의어(들):
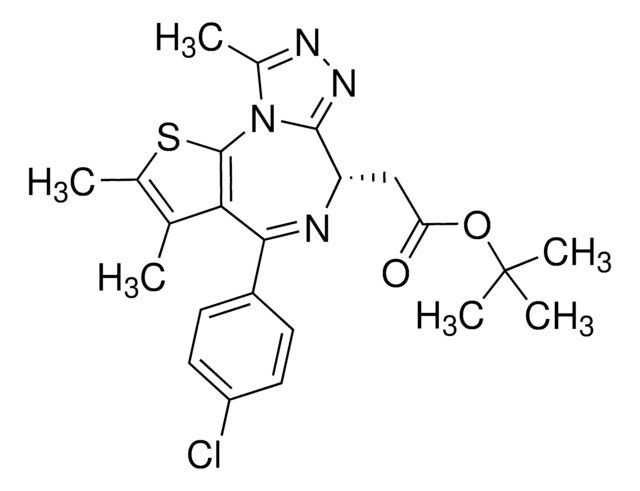
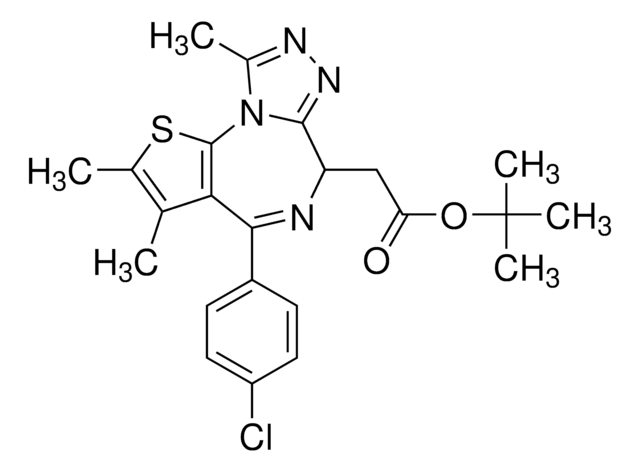
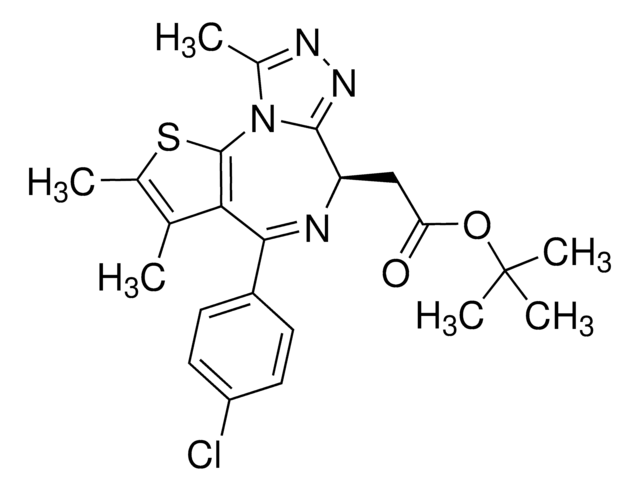
(6S)-4-(4-Chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-acetic acid, (S)-[4-(4-Chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-yl]acetic acid, 2-[(6S,Z)-4-(4-Chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]acetic acid
About This Item
추천 제품
분석
≥95% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 2 mg/mL, clear
저장 온도
2-8°C
SMILES string
[s]1c2c(c(c1C)C)C(=N[C@H](c4[n]2c(nn4)C)CC(=O)O)c3ccc(cc3)Cl
InChI
1S/C19H17ClN4O2S/c1-9-10(2)27-19-16(9)17(12-4-6-13(20)7-5-12)21-14(8-15(25)26)18-23-22-11(3)24(18)19/h4-7,14H,8H2,1-3H3,(H,25,26)/t14-/m0/s1
InChI key
LJOSBOOJFIRCSO-AWEZNQCLSA-N
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.