SRE0006
Thymidine Phosphorylase, recombinant from Escherichia coli
recombinant, expressed in E. coli, Suitable for manufacturing of diagnostic kits and reagents, buffered aqueous solution, ≥500 units/mL
동의어(들):
Gliostatins, PD-ECGF, Thymidine:orthophosphate deoxy-D-ribosyltransferase
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
CAS Number:
MDL number:
UNSPSC 코드:
12352204
NACRES:
NA.54
추천 제품
재조합
expressed in E. coli
Quality Level
형태
buffered aqueous solution
농도
≥500 units/mL
기술
inhibition assay: suitable
색상
colorless to yellow
solubility
soluble
water: soluble
NCBI 수납 번호
UniProt 수납 번호
응용 분야
diagnostic assay manufacturing
배송 상태
wet ice
저장 온도
2-8°C
유전자 정보
Escherichia coli ... deoA(948901)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Research area: CELL SIGNALING
The E. coli thymidine phosphorylase shares 40% sequence homology with the human sequence, which is identical to the angiogenic agent platelet-derived endothelial growth factor. The purified E. coli enzyme has been shown to stimulate blood vessel growth in chick chorioallantoic membrane assays.
The E. coli thymidine phosphorylase shares 40% sequence homology with the human sequence, which is identical to the angiogenic agent platelet-derived endothelial growth factor. The purified E. coli enzyme has been shown to stimulate blood vessel growth in chick chorioallantoic membrane assays.
애플리케이션
Thymidine phosphorylase has been used:
- in a study to evaluate biomarkers for advanced breast cancer patients treated with capecitabine-based first-line chemotherapy.
- in a study to investigate implications for the clinical efficacy of nucleoside analogues.
생화학적/생리학적 작용
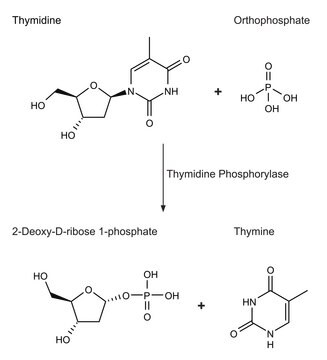
An enzyme that catalyzes the reversible conversion of thymidine to thymine. Thymidine phosphorylase is part of the pyrimidine nucleoside salvage pathway. This pathway allows pyrimidine bases to be recycled for nucleotide biosynthesis, while the pentose 1-phosphates are converted to intermediates of the pentose phosphate shunt and glycolysis. The E. coli thymidine phosphorylase shares 40% sequence homology with the human sequence, which has been found to be identical to the angiogenic agent platelet-derived endothelial growth factor. The purified E. coli enzyme has been shown to stimulate blood vessel growth in chick chorioallantoic membrane assays.
Thymidine phosphorylase catalyzes the reversible conversion of thymidine to thymine. Thymidine phosphorylase is part of the thymidine salvage pathway and pyrimidine nucleoside salvage pathway. This pathway allows pyrimidine bases to be recycled for nucleotide biosynthesis, while the pentose 1-phosphates are converted to intermediates of the pentose phosphate shunt and glycolysis. The enzyme inhibits apoptosis and induces angiogenesis thereby promoting tumor growth and metastatic process. Moreover, thymidine phosphorylase inhibits vascular smooth muscle cell proliferation.
단위 정의
One unit will convert 1.0 μmole each of thymidine and phosphate to thymine and 2-deoxyribose 1-phosphate per min at pH 7.4 at 25°C.
제조 메모
Cloned from E. coli and produced in overexpressing E. coli
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
The dual role of thymidine phosphorylase in cancer development and chemotherapy
Bronckaers A, et al.
Medicinal Research Reviews, 29(6), 903-953 (2009)
Thymidine kinase 1 and thymidine phosphorylase expression in non-small-cell lung carcinoma in relation to angiogenesis and proliferation
Brockenbrough J S, et al.
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 57(11), 1087-1097 (2009)
Structures of native human thymidine phosphorylase and in complex with 5-iodouracil
Mitsiki E, et al.
Biochemical and Biophysical Research Communications, 386(4), 666-670 (2009)
Jen-Chung Ko et al.
Biochemical pharmacology, 88(1), 119-127 (2014-01-23)
Tamoxifen is a triphenylethylene nonsteroidal estrogen receptor (ER) antagonist used worldwide as an adjuvant hormone therapeutic agent in the treatment of breast cancer. However, the molecular mechanism of tamoxifen-induced cytotoxicity in non-small cell lung cancer (NSCLC) cells has not been
Hriday Bera et al.
European journal of medicinal chemistry, 67, 325-334 (2013-07-23)
Thirty-three 1,2,4-triazolo[1,5-a][1,3,5]triazin-5,7-dione and its 5-thioxo analogues were designed and synthesized which contained different substituents at meta- and/or para-positions of 2-phenyl or 2-benzyl ring attached to the fused ring structure. The preliminary pharmacological evaluation demonstrated that the 5-thioxo analogues of 1,2,4-triazolo[1,5-a][1,3,5]triazine
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.