T8951
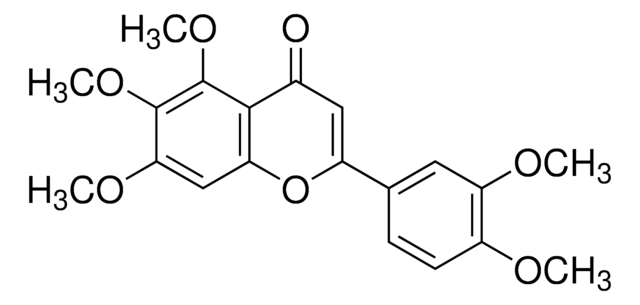
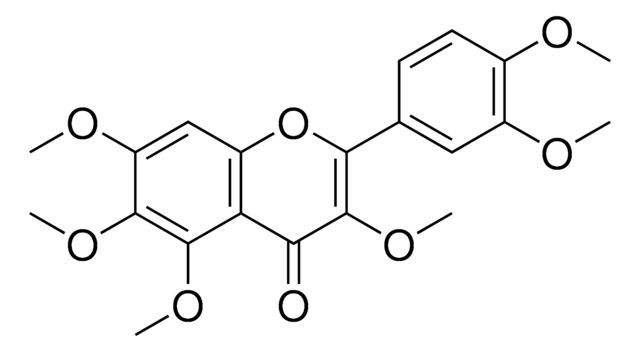
Tangeretin
≥95% (HPLC)
동의어(들):
4′,5,6,7,8-Pentamethoxyflavone, 5,6,7,8-Tetramethoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one, NSC 53909, NSC 618905, Ponkanetin
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H20O7
CAS Number:
Molecular Weight:
372.37
Beilstein:
351695
EC Number:
MDL number:
UNSPSC 코드:
12352205
PubChem Substance ID:
NACRES:
NA.25
추천 제품
Quality Level
분석
≥95% (HPLC)
응용 분야
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
COc1ccc(cc1)C2=CC(=O)c3c(OC)c(OC)c(OC)c(OC)c3O2
InChI
1S/C20H20O7/c1-22-12-8-6-11(7-9-12)14-10-13(21)15-16(23-2)18(24-3)20(26-5)19(25-4)17(15)27-14/h6-10H,1-5H3
InChI key
ULSUXBXHSYSGDT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Tangeretin is a polymethoxyflavone extracted primarily from the peels of citrus fruits like Citrus sinensis and Citrus reticulata.
애플리케이션
Tangeretin has been used as an antioxidant and antifungal agent to test its effects on ferroptosis inhibition, conidial development, and appressorium formation against Magnaporthe oryzae. It has also been used as a co-chemotherapeutics agent to study its cytotoxic effects in combination with 5-Fluorouracil (5-Fu) on WiDr colon cancer cells.
생화학적/생리학적 작용
Tangeretin displays its anti-cancer effects by modulating different pathways in several cancer types in both in-vitro and in-vivo studies. It also exerts antioxidant, anti-inflammatory, and estrogenic properties.
Tangeretin is a flavonoid found in the peel of citrus fruits where it most likely provides natural resistance to fungi. Tangeretin has been shown to counteract tumor promoter-induced inhibition of intercellular communication and to inhibit cell proliferation in several cancer lines.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Combination of Tangeretin and 5-Fluorouracil Modulates Cell Cycle and Induce Apoptosis on WiDr Cells
Indriyani L, et al.,
Indonesian Journal of Cancer Chemoprevention, 3(1), 364-369 (2012)
Jérôme Quintin et al.
Bioorganic & medicinal chemistry letters, 19(1), 167-169 (2008-11-18)
A series of chalcones polyoxygenated on the ring A (with pentamethoxy or 2'-hydroxy-3',4',5',6'-tetramethoxy substitution patterns) was synthesized from tangeretin, a natural Citrus flavonoid. These chalcones were evaluated for their antiproliferative, activation of apoptosis, inhibition of tubulin assembly and antileishmanial activities.
Hideo Satsu et al.
Journal of agricultural and food chemistry, 56(13), 5366-5373 (2008-06-11)
The pregnane X receptor (PXR) is understood to be the key regulator for gene expression of such drug-metabolizing enzymes and transporters as multidrug-resistant protein 1 (MDR1) and the cytochrome P450 (CYP) family. We examined the effect of dietary phytochemicals on
John A Manthey et al.
Journal of agricultural and food chemistry, 59(1), 145-151 (2010-12-08)
Nobiletin (NOB) and tangeretin (TAN), two of the main polymethoxylated flavones (PMFs) in citrus, influence a number of key biological pathways in mammalian cells. Although the impacts of NOB and TAN on glucose homeostasis and cholesterol regulation have been investigated
Hong-Yin Wu et al.
Journal of agricultural and food chemistry, 59(5), 1713-1722 (2011-02-15)
This study investigated the potential effects of natural products ursolic acid (UA) and oleanolic acid (OA) against HBx-mediated tumorigenic activities in vitro and in vivo. HBx transactivated Sp-1 and Smad 3/4 in Huh7 and FL83B hepatocytes and induced cell migration
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.